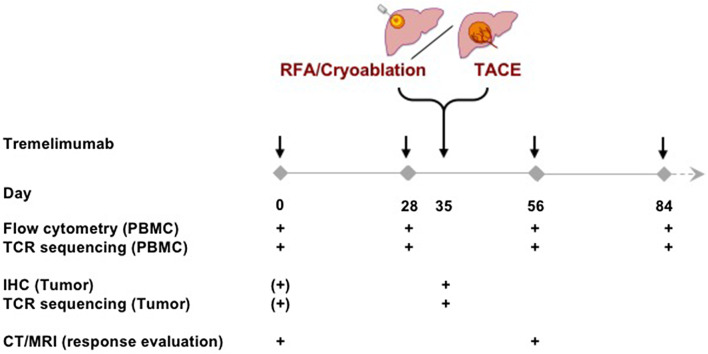
Fig. 1.
Trial design and time points for evaluation of PBMC, tumors and clinical responses. Note that baseline tumor samples may have been obtained at the time of diagnosis. PBMC were harvested on the day of treatment before tremelimumab was given and tumor samples were obtained just prior to loco-regional therapy (IHC immunohistochemistry, RFA radiofrequency ablation, TACE transarterial chemoembolization, PBMC peripheral blood mononuclear cells, TCR T-cell receptor, CT computer tomography, MRI magnet resonance imaging)