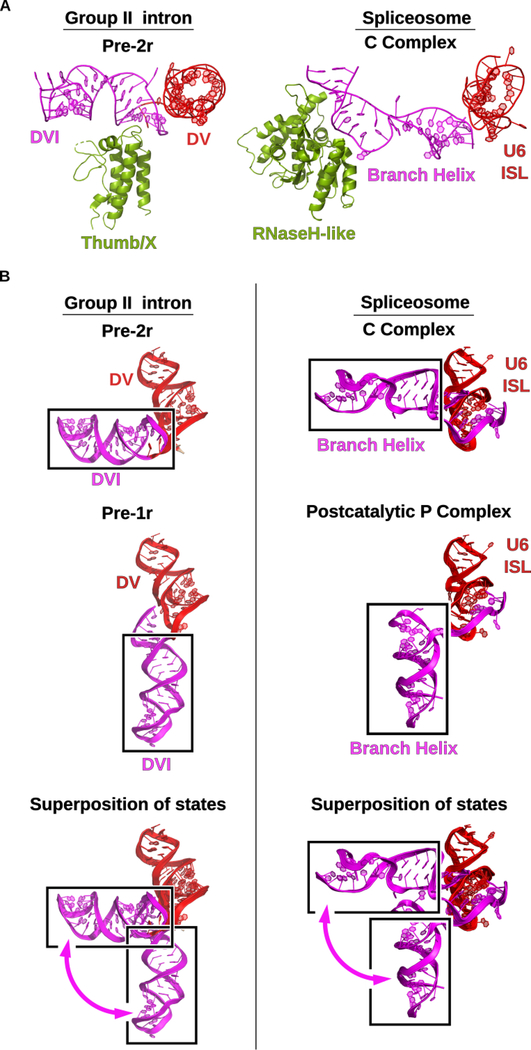
Figure 7.
Group II intron and the spliceosome share mechanistic dynamics. (A) In group II introns the thumb/x domain of the maturase protein promotes splicing by binding to DVI of the RNA and stabilizing the bulged adenosine in the active site to facilitate lariat formation. In the spliceosomal C complex (PDB Accession Code 5LJ5), the RNaseH-like domain of prp8 plays a similar role in binding and transitioning the homologous branch helix through the different stages of splicing (Galej et al., 2016). (B) The DVI helix of the group II intron undergoes a 90° swinging motion between the pre-1r and pre-2r stages of DNA integration. Identical dynamics are observed in the spliceosome in the transition between the C complex (PDB Accession Code 5LJ5) (Galej et al., 2016) and the post-catalytic P (post-P) complex (PDB Accession Code 6QDV) (Fica et al., 2019). The post-P complex and the C complex are structurally homologous to the pre-1r and pre-2r states, respectively. This conserved dynamic motion supports the hypothesis that the spliceosome has evolutionary origins as a group II intron-like retroelement.