Figure 4. Inherent instability of LPL, as judged by hydrogen–deuterium exchange–mass spectrometry (HDX-MS) studies.
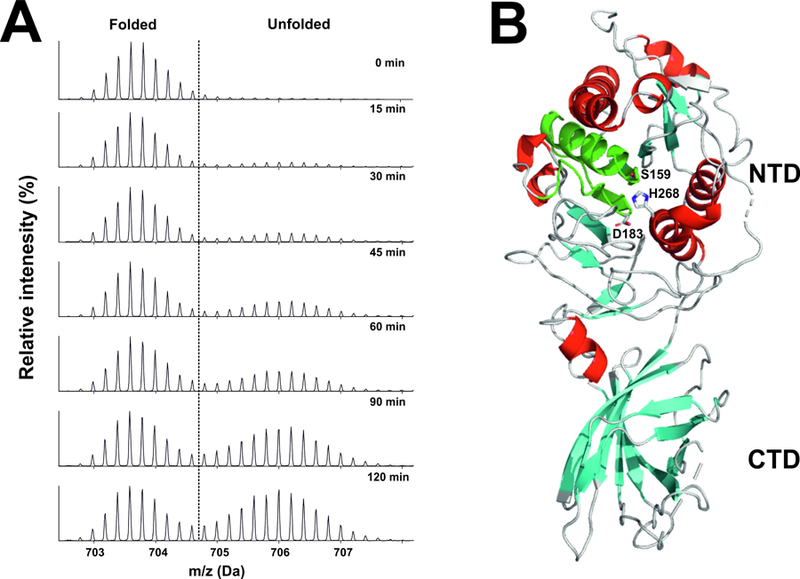
Bovine LPL was incubated at 25°C in deuterium oxide . Panel A shows the emergence of a bimodal isotope envelope of deuterium uptake in a peptic peptide covering the catalytic triad of LPL [from (Mysling et al., 2016a)]. Bimodality of the isotope envelope is a hallmark of protein unfolding (Mysling et al., 2016a; Mysling et al., 2016b). Substantial portions of the amino-terminal catalytic domain of LPL (NTD) exhibited the bimodal isotope envelope, while peptides from the carboxyl-terminal lipid-binding domain (CTD) exhibit a unimodal exchange pattern, reflecting greater stability. Panel B illustrates the position of the catalytic triad peptic peptide (green) in the crystal structure of human LPL [modified from the crystal structure by Birrane and coworkers (Birrane et al., 2019)]. The structure of human LPL is shown as a cartoon representation, with α-helices in red and β-strands in cyan. The location of the three catalytic triad residues (S159, D183, H268) are noted. The structure was visualized with PyMol (Schrödinger) u sing PDB coordinates 6E7K.
