Abstract
Objective
Flow patterns differentially regulate endothelial cell phenotype, with laminar flow promoting vasodilation and disturbed flow promoting endothelial proinflammatory activation. Cystathionine γ-lyase (CSE), a major source of hydrogen sulfide (H2S) in endothelial cells, critically regulates cardiovascular function, by both promoting vasodilation and reducing endothelial activation. Therefore, we sought to investigate the role of CSE in the endothelial response to flow.
Approach and Results
Wild-type C57Bl/6 (WT) and CSE knockout (CSE−/−) mice underwent carotid partial ligation to induce disturbed flow in the left carotid. Additionally, endothelial cells isolated from WT and CSE−/− mice were exposed to either laminar or oscillatory flow, an in vitro model of disturbed flow. Interestingly, laminar flow significantly reduced CSE expression in vitro, and only disturbed flow regions show discernable CSE protein expression in vivo, correlating with enhanced H2S production in WT but not CSE−/− mice. Lack of CSE limited disturbed flow-induced proinflammatory gene expression (ICAM-1, VCAM-1) and macrophage infiltration, and CSE−/− endothelial cells show reduced NF-κB activation and proinflammatory gene expression in response to oscillatory flow in vitro. Additionally, CSE−/− mice showed reduced inward remodeling following carotid ligation. CSE−/− mice show elevated vascular nitrite levels (measure of nitric oxide (NO)) in the unligated carotids, suggesting an elevation in baseline NO production, and the NO scavenger c-PTIO normalized the reduced inward remodeling, but not inflammation, of ligated carotids in CSE−/− mice.
Conclusions
CSE expression in disturbed flow regions critically regulates both endothelial activation and flow-dependent vascular remodeling, in part through altered NO availability.
Keywords: sulfide, shear stress, disturbed flow, vascular remodeling, cystathionine γ-lyase (CSE), Basic, Translational, Clinical Research - Vascular Biology
Introduction
Blood vessels are constantly exposed to mechanical stimuli from circulating blood. The movement of blood exerts a frictional force, namely fluid shear stress, parallel to the vascular wall. Patterns of blood flow in circulation vary along the hierarchical tree of blood vessels. In most arterial regions, blood flow is unidirectional, high velocity, and laminar. However, bifurcations, branch points, curvatures in vessels cause disturbed flow profiles characterized by low velocity shear stress and changes in flow direction. These distinct patterns of shear stress differentially regulate vascular heterogeneity and homeostasis. While high velocity, laminar shear maintains endothelial cells quiescence, low velocity, oscillatory shear activates endothelial cells resulting in enhanced monocyte recruitment1. Laminar flow and disturbed flow are sensed by the endothelial cell layer resulting in disparate cellular responses. Exposing endothelial cells to oscillatory flow activates nuclear factor κ B (NF-κB), enhances expression of adhesion molecules, and induces cell proliferation and apoptosis, while laminar flow inhibits inflammatory signaling and reduces endothelial cell turnover2–4. In addition to these flow patterns, vessels also respond to changes in blood flow. Elevated flow induces transient vasodilation and outward remodeling if the changes become chronic, whereas reductions in flow result in transient vasoconstriction and inward remodeling should flow reductions be sustained.
Hydrogen Sulfide (H2S) is an important signaling molecule in the cardiovascular system where cystathionine γ-lyase (CSE) is a major source of it5. H2S and CSE play critical roles in endothelial function and vascular health. Our previous studies have shown that CSE derived polysulfides, oxidative products of H2S, promotes angiogenesis in ischemic limbs and increases endothelial permeability6, 7. CSE knockout mice were also shown to have increased atherosclerotic plaque formation following high fat diet, while CSE transgenic mice on the apolipoprotein E knockout background are protected against plaque formation8, 9. Although, vascular remodeling is the basis of neointimal formation and stenosis, it can be largely affected by dyslipidemia in models of atherosclerosis. It is not known whether CSE is involved in the physiological vascular adaptive response to shear stress independent of lipid metabolism disorders. In this study, we used CSE knockout mice on the C57BL/6J to investigate the role of CSE in flow mediated vascular remodeling.
Material and Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Animals
All mice used in this study were housed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Studies were approved by LSU Health-Shreveport institutional animal care and use committee. Partial carotid ligations were performed on 12–18 week-old male C57BL/6J wild-type and CSE knockout mice. As we described in our previous study, the left external carotid, internal carotid and occipital arteries were ligated with suture, sparing the superior thyroid artery, while the right side was left intact as the non-ligated control3. Ultrasound measurements were taken under anesthesia with a VisualSonics VEVO 3100 system before and one or six day(s) after ligation to confirm the reduction in blood flow. Mice were euthanized 2 days and 7 days after ligations to collect their carotid arteries. Endothelial mRNA samples were obtained 2 days post-ligation by flushing TRIzol® through the artery. The 7-day samples were used for immunohistochemistry and the biochemical measurements.
Cell culture and shear stress
Human aortic endothelial cells were purchased from Lonza and maintained at 37 °C in MCDB 131 media (Sigma, M8537) with 10% fetal bovine serum, 2 mM glutamine, 10 U/ml penicillin, 10μg/ml streptomycin, 60 μg/mL heparin and 170 μg/ml bovine brain extract. Mouse aortic endothelial cells (MAECs) were isolated from three wild-type or CSE knockout mice as previously described6. Briefly, aortic endothelial cells were collected by Matrigel invasion (Corning, 354234) and enriched with Dynabeads (Invitrogen, 11205D) coupled with a CD105 antibody (eBioscience, 13–1051-85). The resultant endothelial cells were immortalized with a temperature sensitive large T-antigen. MAECs were cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 10 U/ml penicillin, 10μg/ml streptomycin. The shear stress experiments were performed using parallel plate flow chambers and peristatic pumps as previously reported10, 11. Cells were exposed to either laminar flow (12 dynes/cm2) or oscillatory flow (±5 dynes/cm2 with 1 dynes/cm2 superimposed for nutrient/waste exchange). After shear treatments, cells were lysed directly in 2X laemmli buffer for western blotting or fixed in 4% formaldehyde for immunocytochemistry.
Immunoblotting
Proteins in the cell lysate were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% dry milk in TBS with 0.1% Tween-20 and blotted using antibodies listed in the Major Resource Table in the Supplemental Material.
Immunocytochemistry
Fixed endothelial cells were permeabilized with 0.1% Triton-X in PBS and stained for the NF-κB p65 subunit (1 μg/ml, Santa Cruz, Sc-109). The primary antibody was probed with Alexa Fluor 488 conjugated secondary antibody. Nuclei were visualized using DAPI staining. Fluorescent images were taken from at least five random fields on each slide.
Tissue staining
Carotid tissue was fixed with 4% formaldehyde and embedded in paraffin. 5-μm sections 150–450 μm proximal from the ligation site were used for immunohistochemistry and Russell-Movat Pentachrome staining as previously described12. Carotid medial thickness and circumference with Nikon Element software. Lumen size is calculated according to circumference. Primary antibodies used for immunostaining are listed in the Major Resource Table in the Supplemental Material.
qRT-PCR
mRNA was isolated from TRIzol® samples according to the manufacture’s instruction. The iScript cDNA synthesis kit (Bio-rad, 1708890) was used to transcribe mRNA. qRT-PCR was performed using SYBR® Green Master Mix (Bio-rad, 1708882), and gene expression was quantified using the ddCt method. All genes of interest were normalized to the housekeeping genes B2M and RPL13A. The primers used in PCR reactions are listed in the Major Resource Table in the Supplemental Material.
Sulfide measurement
H2S and its metabolites were measured using a monobromobimane (MBB) based method as we previously reported13. Carotid arteries were homogenized in 250 μl reaction buffer (0.1mM diethylenetriaminepentaacetic acid in 100mM Tris buffer, pH 9.5) with 0.1% Triton-X. H2S were derivatized with MBB. The pools of sulfane sulfurs and acid labile sulfurs were released from the sample in reducing and acidic environments respectively, followed by MBB derivatization. The resultant product sulfide dibimane was quantified by reverse phase high performance liquid chromatography.
Nitric oxide bioavailability
Nitric oxide (NO) metabolites (NOx) were measured as we previously reported14. Carotid arteries were homogenized in 250μl NO preservation buffer (1.25 M potassium ferricyanide, 56.9 mM N-ethylmaleimide, 6% NP-40 in PBS). Nitrite is reduced with the triiodide method. NO is measured by the ozone based chemiluminescent assay (Sievers Nitric Oxide Analyzer 280i).
Statistical Analysis
Data were shown as mean ± standard error of the mean. Data was tested for normality using GraphPad Prism software and analyzed for statistical significance using either parametric (Student t-test, Two-way ANOVA with Tukey’s post hoc test) or non-parametric tests (Mann-Whitney U test) as indicated in the figure legends. P values < 0.05 were considered as statistical significance.
Results
Flow patterns regulate endothelial CSE expression
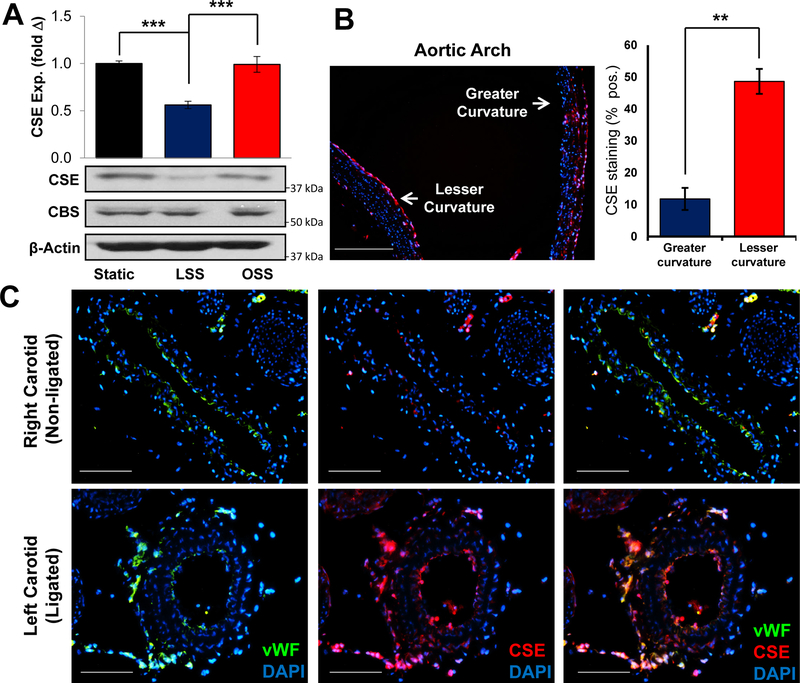
Flow patterns critically regulate endothelial activation and endothelial-dependent vasodilation15–17, where H2S signaling has been shown to be important7, 18, 19. Since CSE is the predominant source of sulfide in endothelial cells, we asked whether its expression is regulated by flow. Human aortic endothelial cells were exposed to long-term (18 hrs) laminar or oscillatory flow, and CSE expression was assessed by Western blotting. Surprisingly, laminar flow significantly reduced CSE expression compared to oscillatory flow (Figure 1A). In comparison, the expression of cystathionine β-synthase (CBS), another sulfide producing enzyme reported to regulate endothelial function20, was not altered by flow patterns. To study the effects of shear stress on CSE expression in vivo, we analyzed CSE staining the aortic arch, which experiences high velocity laminar flow on the greater curvature and disturbed flow on the lesser curvature. Consistent with our cell culture models, the aortic lesser curvature showed higher CSE expression compared to the greater curvature (Figure 1B), suggesting that endogenous sites of laminar and disturbed flow show a similar flow pattern-specific CSE expression. To validate this in an acute model of flow disturbances, we performed partial carotid ligation to induce low, oscillatory flow in the left carotid arteries, with the right carotid serving as an internal control for high laminar flow3. Alterations in flow dynamics were verified by ultrasound after the surgery, showing the flow rate was reduced 80% in the left carotid artery (Supplemental Figure I). As shown in Figure 1C, CSE expression was increased in the ligated carotid artery compared to the contralateral side. Specificity of this staining pattern was verified using IgG controls, secondary antibody-only controls, and CSE knockout tissue (Supplemental Figure II). Importantly, the increased CSE expression largely colocalized with endothelial cells (von Willebrand factor (vWF)-positive) in the vessel intima and adventitia. The microvessels in adventitia, known as vasa vasorum, appeared to undergo expansion in the ligated vessels. While CSE is known to regulate angiogenesis in a variety of models21, we did not observe any effect of CSE deletion on vasa vasorum expansion in this model (Supplemental Figure III).
Figure 1. Shear stress regulates CSE expression.
Human aortic endothelial cells (HAECs) were exposed to LSS or OSS for 16 hours. (A) CBS and CSE expression was assessed by Western blotting. Representative images are shown. (n=5, *** p<0.001 by One-Way ANOVA). CSE protein expression was assessed by immunostaining at sites of laminar (greater curvature) or disturbed flow (lesser curvature). (scale bar = 200 μm, n=5, ** p<0.01 by Mann-Whitney U test). (C) Partial carotid ligation was performed in WT mice to induce disturbed flow in the ligated left carotid compared to the unligated right carotid. (scale bar = 100 μm, n=7).
Flow patterns and sulfide metabolism
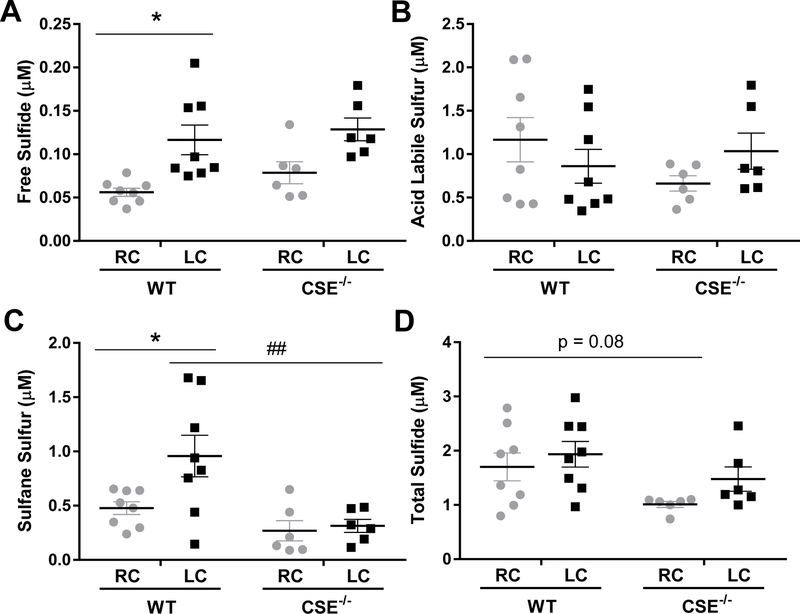
Free H2S can be metabolized to sulfane sulfurs and acid labile sulfurs, which serve as biological reservoirs of H2S. Since laminar flow-induced CSE suppression is lost in areas of disturbed flow, we wanted to know whether this led to the elevation of H2S and its metabolites. Sulfide pools from the carotid arteries were measured seven days after ligation (Figure 2). In wild-type mice, both free H2S and sulfane sulfurs were increased by the ligation, while the acid labile pool was not affected. CSE knockout mice showed a complex change of sulfur species. In non-ligated carotid arteries, CSE knockout led to a trend toward basal reduction of sulfane sulfur content but an unexpected increase of free sulfide, although neither reached statistical significance. Acid labile sulfur was unchanged. Of particular importance, the augment in sulfane sulfur following ligation was blunted in CSE knockout mice, while the change of free sulfide was not affected. Total sulfide levels showed a negative trend in the CSE knockout mice but did not reach statistical significance (p=0.08). Together, these data suggest disturbed flow increases CSE-derived sulfane sulfur, which may be involved in flow mediated vascular responses.
Figure 2. Partial Ligation Enhances Free and Sulfane Sulfur in WT but not CSE−/− Carotid Arteries.
Distinct sulfide pools, including free H2S (A), acid labile sulfur (B), sulfane sulfur (C), and total sulfide (D) were measured by monobromobimane 7 days after partial carotid ligation in WT and CSE−/− mice. (n=6–8, * p<0.05 comparing RC to LC, ## p < 0.01 comparing WT to CSE−/− by Two-Way ANOVA).
CSE and flow-mediated vascular inflammation
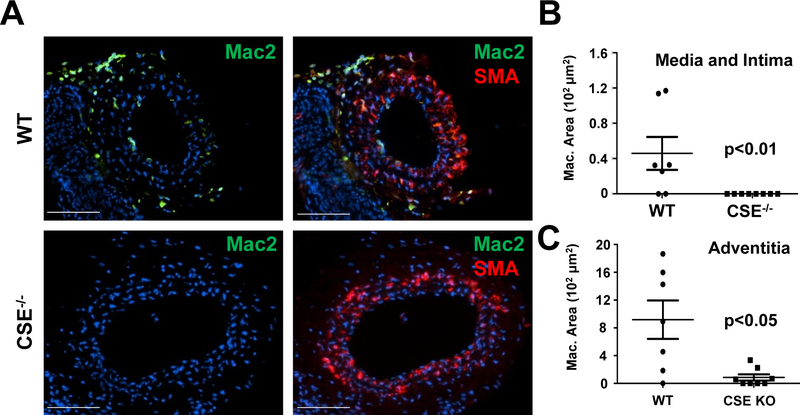
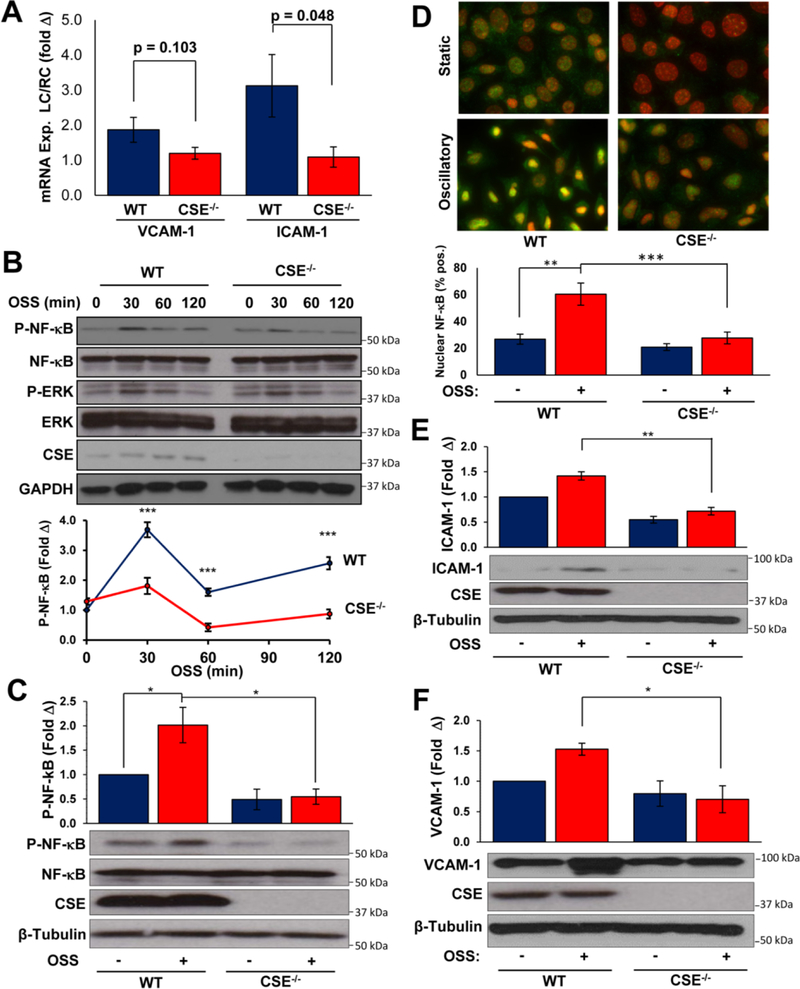
Disturbed flow patterns promote endothelial proinflammatory activation through enhanced NF-κB signaling, whereas laminar flow limits endothelial activation22. In wild-type mice, the partial carotid ligation increased Mac-2 positive macrophages seven days after the surgery (Figure 3A–C), whereas the contralateral right carotids were devoid of macrophage staining (Supplemental Figure IV). Surprisingly, this macrophage recruitment was completely blunted in CSE knockout mice (Figure 3A–C). To study endothelial pro-inflammatory gene expression, we isolated intimal mRNA two days after ligation as previously described3, 23. Vascular endothelial adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) are normally up regulated on the surface of endothelial cells by disturbed flow to recruit leukocytes from the circulation. Up regulation of VCAM-1 and ICAM-1 mRNA is blunted in CSE knockout intima (Figure 4A). To assess the proinflammatory response to flow in vitro, we analyzed NF-κB activation and proinflammatory gene expression in response to acute (< 2 hours) or chronic (18 hrs) oscillatory flow. CSE KO endothelial cells showed reduced NF-κB activation, as assessed by phosphorylation of the p65 subunit (hereafter NF-κB), in response to acute oscillatory flow (Figure 4B). However, ERK1/2 activation by oscillatory flow was unaffected (Supplemental Figure V), suggesting that CSE is not required for all flow-induced responses. Consistent with this pattern, chronic oscillatory flow-induced NF-κB activation, assessed by NF-κB phosphorylation (Figure 4C) and nuclear translocation (Figure 4D), was similarly blunted in CSE KO endothelial cells. In addition, CSE KO endothelial cells show diminished oscillatory flow-induced ICAM-1 expression (Figure 4E) and VCAM-1 expression (Figure 4F). Thus, CSE critically regulates flow-induced NF-κB signaling to drive ICAM-1/VCAM-1 expression and macrophage recruitment.
Figure 3. Absence of macrophages in ligated CSE−/− carotids.
(A) 7 days after partial carotid ligation in WT and CSE−/− mice, mouse carotid arteries were immunostained for macrophages (Mac-2) and smooth muscle actin (SMA). (B/C) Macrophages in (B) tunica intima, media, and (C) adventitia were quantified by Mac-2 positive area (scale bar = 100 μm, n=7–8, p<0.05 by (B) Mann-Whitney U Test and (C) Students T-test).
Figure 4. Shear-induced NF-kB Activation and ICAM-1 expression are reduced in CSE−/− endothelium.
(A) Intimal mRNA was collected 48 hours after partial ligation and assessed for VCAM-1 and ICAM-1 expression. n=4–8. (B) NF-κB phosphorylation following short-term oscillatory shear stress (OSS, 0 to 120 min) was prevented in CSE−/− MAECs. n=4. (C/D) NF-κB activation following chronic OSS (16 hours) was assessed by (C) phosphorylation and (D) nuclear translocation in WT and CSE−/− MAECs. For nuclear translocation, at least 80 cells were scored for nuclear NF-κB staining per condition within each experiment. n=4. (E/F) Chronic OSS-induced (E) ICAM-1 and (F) VCAM-1 expression was assessed by Western blotting. Representative images are shown. n=4. * p<0.05, ** p < 0.01, *** p<0.001 by Students T-test (A) or Two-Way ANOVA (B-F).
CSE and flow-dependent vascular remodeling
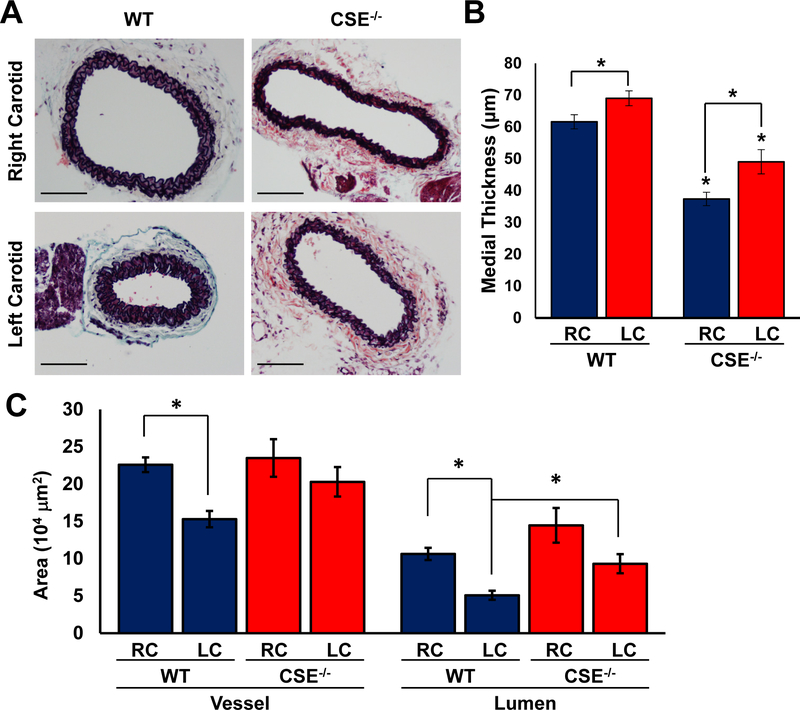
Reductions in blood flow cause inward vascular remodeling to reduce lumen diameter and maintain shear forces within a physiological range. In the absence of hypercholesterolemia, the reductions in blood flow observed in the partial carotid ligation model results in inward remodeling with reduced lumen volume, medial thickening, and adventitial expansion24, 25. Since endothelial CSE was up-regulated in vessels with low/disturbed flow, we examined whether CSE expression affects the remodeling of these vessels in response to reduced flow. In wild-type mice, the thickness of tunica media was increased seven days after the partial carotid ligation (Figure 5A–B). In comparison, CSE knockout mice exhibited reduced medial thickness compared to the wild-type mice in both ligated and non-ligated carotid arteries (Figure 5A–B). Meanwhile, partial carotid ligation resulted in a reduced overall vessel area and smaller lumen size in the wild-type mice. However, CSE knockout mice did not show a significant change in vessel or lumen area following partial ligation (Figure 5A, C), and lumen area was significantly larger in CSE knockout mice. While these data suggest that CSE deficient mice show defective inward remodeling, CSE deficiency did not fully prevent the ligation induced medial thickening (Figure 5B). Therefore, CSE contributes to the flow-mediated vascular remodeling, whereas the lack of CSE significantly blunts this process.
Figure 5. Impaired vascular remodeling in CSE−/− carotids.
(A) Movat’s stain was performed 7 days after carotid partial ligation. (B-C) Medial thickness, vessel and lumen areas were quantified (scale bar = 100 μm, n=7–8, p<0.05 by Two-Way ANOVA).
Flow patterns and NO metabolism
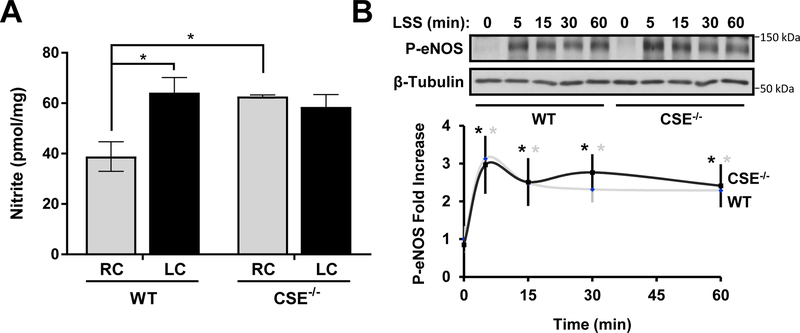
H2S has been shown to interact with NO signaling on multiple levels21. Here we examined NO availability in the carotid artery samples following partial carotid ligation (Figure 6A). Ligation increased NO metabolites (NOx) concentration in wild-type mice but not CSE knockout mice. Meanwhile, CSE knockout mice showed an elevated basal level of NOx in carotid artery tissue. These patterns correlate with the change of free H2S and sulfane sulfurs. H2S can increase NO synthesis by activating eNOS activity26, 27. In cell culture, short-term laminar shear activates eNOS activity by the phosphorylation of S1176. However, the eNOS phosphorylation by shear was not different between wild type and CSE knockout MAECs (Figure 6B).
Figure 6. Increased NO Availability in CSE−/− Carotid Arteries.
(A) Nitrite levels in carotid arteries were measured by triiodide chemiluminescence 7 days after partial ligation (n=5–7, p<0.05 by Two-Way ANOVA). (B) LSS induced eNOS phosphorylation was not affected in CSE−/− MAECs (n=4 * indicated time points vs static condition, p<0.05 by Two-Way ANOVA).
The role of NO in the remodeling of CSE deficient carotid artery to disturbed flow
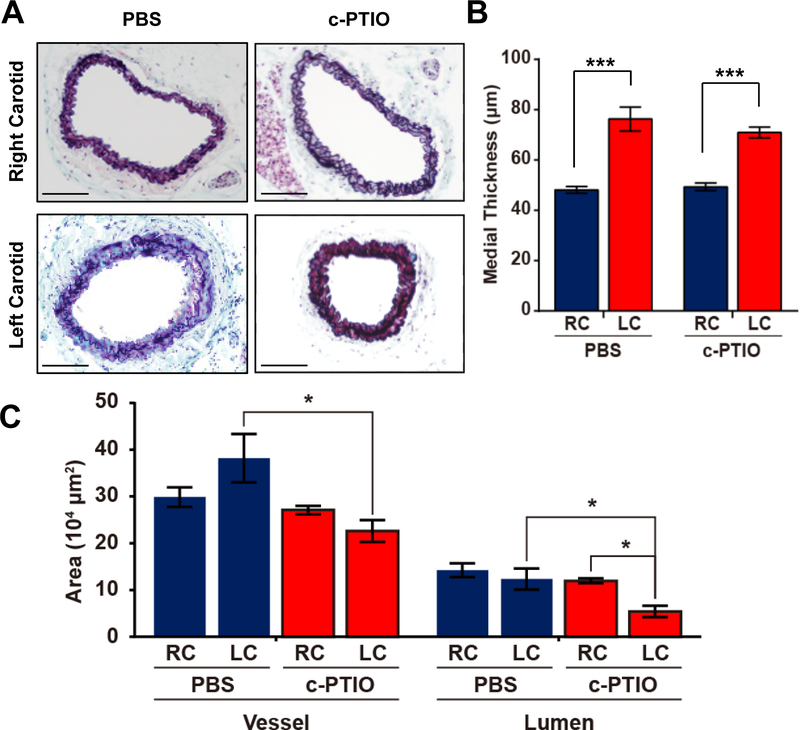
CSE knockout carotid arteries exhibited the higher level of NOx and the inability to reduce lumen size under reduced flow. To investigate whether this dilated phenotype was due to the excessive NO in the vasculature, we treated CSE knockout mice with c-PTIO, an NO scavenger, before and after the ligation. To verify NO scavenging, we measured plasma nitrite levels and observed a significant reduction following cPTIO treatment (Supplemental Figure VI). The ligated carotid artery in CSE knockout mice regained the ability to reduce lumen diameter with the c-PTIO treatment (Figure 7C). However, NO scavenging did not alter the medial thickening induced by ligation. Furthermore, CPTIO treatment did not significantly enhance macrophage recruitment following partial carotid ligation in CSE KO mice (Supplemental Figure VIIa–c) and did not restore oscillatory flow-induced NF-κB activation or ICAM-1 expression in CSE KO endothelial cells (Supplemental Figure VIId–e). These data suggest that the elevated NO bioavailability in CSE knockout carotid arteries contributes to the dilated phenotype but not the increased medial thickness or the reduced inflammation.
Figure 7. C-PITO prevented the dilated carotids in CSE−/− mice.
CSE−/− mice were treated with c-PTIO (1 mg/kg) for consecutive 10 days. Partial carotid ligation was performed on Day 4. Carotids were collected after another 7 days. (A) Movat’s stain was performed 7 days after carotid partial ligation in CSE−/− mice. (B-C) Medial thickness, vessel and lumen areas were quantified (scale bar = 100 μm, n=6–8, p<0.05, *** p<0.001 using Two-Way ANOVA.
Discussion
Blood flow critically regulates vascular homeostasis and function. In embryonic development, maturation of normal blood vessels requires hemodynamic force28. In adults, shear stress regulates vasodilation, vascular remodeling, and susceptibility to atherosclerotic plaque formation. In large arteries such as aorta and carotid arteries, the vascular wall is mostly exposed to a high velocity laminar flow. This laminar flow is beneficial for vascular health by suppressing inflammatory genes, enhancing eNOS activity and nitric oxide availability, and reducing reactive oxidative species29. In comparison, disturbed flow in vessel curvatures, branch points, and bifurcations stimulates an atherosusceptible endothelial phenotype, with reduced NO production, enhanced oxidant stress, and increased pro-inflammatory gene expression.
As the predominant source of H2S in the cardiovascular system, CSE critically regulates multiple aspects of vascular function, including endothelial permeability and ischemic vascular remodeling6, 7. In this study, we found CSE expression is regulated by shear stress. In the lesser aortic curvature and the ligated carotid artery where blood flow is disturbed, endothelial CSE is highly expressed (Figure 1). However, in vitro flow models suggest that laminar flow-induced CSE downregulation plays a dominant role in this differential expression pattern. We also noticed that microvasculature in the vasa vasorum shows strong CSE expression in both ligated and non-ligated carotid arteries, and this staining increased upon vasa vasorum expansion after vessel ligation. These data suggest that CSE may play a larger role in regulating microvascular function under physiological conditions, as it is only expressed in large vessel endothelial cells at atherosclerosis-prone sites. Interestingly, NF-κB critically regulates CSE expression30, and laminar flow suppresses NF-κB- dependent proinflammatory gene expression 31, suggesting a potential NF-κB-dependent regulatory mechanism.
Increased CSE expression in the ligated carotid arteries is associated with elevated sulfane sulfur content, which is blunted by genetic CSE deficiency (Figure 2). Sulfane sulfur species include oxidative products of H2S such as persulfides and polysulfides, which can not only serve as a biological reservoir for H2S but are highly reactive themselves. These reactive sulfur species, like reactive oxygen species, can modify protein cysteines and subsequent protein functions32. Mounting evidence suggests it is sulfane sulfurs that mediate many biological effects originally attributed to free H2S21. It is also shown that, in addition to H2S oxidation, H2S producing enzymes such as CSE can be a direct source of sulfane sulfur33. In the current study, we showed that CSE induced by disturbed flow favored the production of sulfane sulfur rather than free H2S, since the latter was not affected by the absence of CSE. However, it is not clear whether this is due to increased oxidative stress under disturbed flow or preferentially regulated by CSE activity toward sulfane sulfur generation.
H2S has been ascribed both anti-inflammatory and proinflammatory functions, potentially due to the dose or pool of sulfur species involved34. CSE deletion prevented the recruitment of macrophages in the ligated carotid artery possibly by suppressing the activation of NF-κB pathway and the expression of adhesion molecules in endothelium (Figure 3–4). NF-κB plays a critical role in disturbed flow-associated inflammation and is critical for macrophage recruitment at atheroprone regions29, 35. However, the role of CSE and H2S in NF-κB regulation may depend upon the cell type, spatial signaling, and dose. In endothelial cells, addition of exogenous H2S prevented TNF-α induced NF-κB activation and expression of ICAM-1 and VCAM-118. While the inhibitory effect of exogenous H2S on NF-κB phosphorylation, nuclear translation, and DNA binding was shown to depend upon p65 sulfhydration at Cys38, enhanced H2S production by endogenous CSE promotes NF-κB activation, DNA binding, and anti-apoptotic gene expression through a similar Cys38 sulfhydration-dependent mechanism36, 37. Consistent with a role for endogenous CSE-derived H2S in proinflammatory responses, CSE knockdown significantly diminished LPS-induced NF-κB activation in macrophages38, and CSE knockout mice show reduced NF-κB activity and inflammation in response to sepsis39. However, CSE knockout mice also show enhanced inflammation in atherosclerosis models9, suggesting that this anti-inflammatory effect may be context-dependent.
Previously, CSE has been shown to be protective against atherosclerosis8, 9, 40. Following 12-week high fat diet, CSE knockout mice had increased atherosclerotic lesion area on both wildtype (C57BL/6J/129SvEv) and apolipoprotein E knockout background9. The accelerated atherosclerosis was inhibited by either exogenous H2S or global CSE over-expression on CSE knockout mice8, 9. Additionally, exogenous H2S reduced ICAM-1 expression in TNF-α treated endothelial cells in culture41. Collectively, these results demonstrate anti-inflammatory roles of CSE and H2S. However, the development of atherosclerosis involves multiple factors such as hyperglycemia, hyperlipidemia and leukocyte recruitment, where the role of CSE and H2S can be complicated. In our model, we focused on the physiological roles of CSE on vascular remodeling in adaption to different flow patterns.
With regards to this matter, Yang et al showed increased intimal formation in CSE deficient mice after 4-week carotid artery ligation40. They also showed that smooth muscle cells isolated from these mice exhibited increased oxidative stress and ICAM-1 expression. However, it is well known that distinct mouse strains have different vascular remodeling phenotypes42. The CSE deficient mice used in early studies were on the C57BL/6J/129SvEv mixed background. 129/SvEv strain has two copies of renin genes, which may contribute to their age dependent hypertension9. Additionally, hypertension predisposes the mice to atherosclerosis, making it difficult to study the role of disturbed flow in vascular remodeling. In our current study, we established fully congenic CSE genetic mutation on the C57BL/6J background, which do not develop spontaneous hypertension43. Moreover, our carotid ligation lasted for only one week, since we focused on early vascular response to disturbed flow. In contrast to CSE knockout mice on a mixed background, we saw CSE deficiency partially prevented ligation induced medial thickening, indicating CSE contributes to, rather than protects against, the inward vascular remodeling induced by disturbed flow (Figure 5).
It is thought that blood vessels have a set-point of shear stress. The alteration of shear stress results in the change of vessel diameter to restore the original magnitude of shear stress44. However, this regulation mechanism is compromised in the CSE knockout carotid artery that remained dilated under reduced shear following ligation (Figure 2). Endothelium derived NO and eNOS activity is essential in the regulation of vascular tone. It is also well known that eNOS is phosphorylated at Ser 1177 and activated by shear stress45. Previous studies have shown that H2S and NO work in a synergetic fashion26, 27. One study showed that silencing CSE in HUVECs attenuated eNOS phosphorylation at Ser 1177 under shear stress46. Interestingly, endothelial cells isolated from CSE knockout mice did not show compromised eNOS activation by shear stress (Figure 6 B). In vivo, carotid ligation increased the NOx level in the wild-type mice, which is in concurrent with macrophage infiltration ligated carotid arteries, suggesting the involvement of iNOS (Figure 6 A). In comparison, the NOx level was not altered by ligation in the CSE knockout mice. Instead, CSE knockout showed basally elevated NOx. This upregulation of basal NO availability is not likely a contribution of iNOS, since macrophage infiltrates were absent in the CSE knockout carotid artery. On the contrary, nNOS may play a compensatory role in CSE knockout vessels. As a nitric oxide synthase mainly found in the nervous system, nNOS in innervating nerve fibers can be important for normal vascular function47, 48. Although it is undetectable in normal vessels, nNOS is up-regulated in vascular smooth muscle cells and endothelial cells and plays a protective role under atherosclerosis49–51.
Aside from a possible role of nNOS, it has been reported that sulfide and its metabolites (e.g. hydropersulfides) can react with NO to form a range of chemical products, including unique S-N hybrid species such as nitroso-persulfide (SSNO) with concomitant abilities to regulate vascular reactivity52, 53. Given that CSE deficient vessels had significantly blunted sulfane sulfur, it is possible that NOx species accumulate due to the absence of a substantial chemical reaction pathway as hydropersulfide or polysulfide levels are known to exist in micromolar quantities54. Future studies are required to confirm the source of NO in CSE knockout vessels and to find out how CSE is regulating it. Regardless of its source, the increase in NO availability prevented the carotid artery from constriction following ligation as the NO scavenger CPTIO was able to rescue the dilated phenotype (Figure 7). However, NO scavenging did not change the thickness of tunica media, suggesting medial thickening and lumen narrowing are differentially regulated in CSE knockout mice.
In conclusion, CSE plays an important role in flow induced vascular remodeling. CSE expression and sulfane sulfur production are enhanced by disturbed flow in conduit vessels. The enhanced CSE expression correlates with macrophage recruitment to these areas, which is possibly through a NF-κB dependent pathway. CSE knockout mice exhibits a complex change of vascular remodeling under disturbed flow, including reduced medial thickening and inability to narrow lumen size. The dilated phenotype in CSE knockout mice was NO dependent but not due to eNOS dysfunction.
Supplementary Material
Highlights.
Vascular CSE expression and sulfane sulfur are enhanced by disturbed flow.
Enhanced CSE derived sulfane sulfur under disturbed flow promotes endothelial cell activation, macrophage recruitment and vascular inward remodeling.
CSE deficiency does not affect flow-induced eNOS phosphorylation but enhances nitrite levels in the carotid artery (both ligated and non-ligated) resulting in a dilated vascular phenotype in an NO dependent manner.
Acknowledgments
Sources of funding
Predoctoral fellowship from Malcolm Feist Cardiovascular Research Endowment, LSUHSC-S to S.Y., A.Y.J., and Z.A.Y. and Postdoctoral Fellowship to M.A.; AHA Predoctoral Fellowship to A.Y.J.; NIH grants HL098435 and HL133497 to A.W.O.; HL113303 to C.G.K., and GM121307 to C.G.K. and A.W.O.
Abbreviations
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- eNOS
endothelial nitric oxide synthase
- H2S
hydrogen sulfide
- ICAM-1
intercellular adhesion molecule 1
- MAEC
mouse aortic endothelial cell
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NOx
nitric oxides, nitric oxide metabolites
- SMA
smooth muscle actin
- VCAM-1
vascular cell adhesion molecule 1
- vWF
Von Willebrand factor
References
- 1.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe J. Pkczeta mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. J Cell Biol. 2011;193:867–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Green J, Yurdagul A Jr., Albert P, McInnis MC, Orr AW. Alphavbeta3 integrins mediate flow-induced nf-kappab activation, proinflammatory gene expression, and early atherogenic inflammation. Am J Pathol. 2015;185:2575–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao X, Lu C, Frangos JA. Mechanism of temporal gradients in shear-induced erk1/2 activation and proliferation in endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:H22–29 [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2s as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan S, Pardue S, Shen X, Alexander JS, Orr AW, Kevil CG. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol. 2016;9:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine gamma-lyase regulates arteriogenesis through no-dependent monocyte recruitment. Cardiovasc Res. 2015;107:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung SH, Kwok WK, To KF, Lau JY. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (cse) gene. PLoS One. 2014;9:e113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhotak S, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534 [DOI] [PubMed] [Google Scholar]
- 10.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates nf-kappab activation by flow: A potential role in atherosclerosis. J Cell Biol. 2005;169:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr AW, Hahn C, Blackman BR, Schwartz MA. P21-activated kinase signaling regulates oxidant-dependent nf-kappa b activation by flow. Circ Res. 2008;103:671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yurdagul A Jr., Green J, Albert P, McInnis MC, Mazar AP, Orr AW. Alpha5beta1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1362–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen X, Kolluru GK, Yuan S, Kevil CG. Measurement of h2s in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015;554:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, Kevil CG. Plasma free h2s levels are elevated in patients with cardiovascular disease. J Am Heart Assoc. 2013;2:e000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yurdagul A Jr., Orr AW. Blood brothers: Hemodynamics and cell-matrix interactions in endothelial function. Antioxid Redox Signal. 2016;25:415–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLenachan JM, Vita J, Fish DR, Treasure CB, Cox DA, Ganz P, Selwyn AP. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation. 1990;82:1169–1173 [DOI] [PubMed] [Google Scholar]
- 17.Butler PJ, Weinbaum S, Chien S, Lemons DE. Endothelium-dependent, shear-induced vasodilation is rate-sensitive. Microcirculation. 2000;7:53–65 [PubMed] [Google Scholar]
- 18.Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011;6:e19766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S, Chakraborty PK, Xiong X, Dwivedi SK, Mustafi SB, Leigh NR, Ramchandran R, Mukherjee P, Bhattacharya R. Cystathionine beta-synthase regulates endothelial function via protein s-sulfhydration. FASEB J. 2016;30:441–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan S, Shen X, Kevil CG. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal. 2017;27:634–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudic RD, Bucci M, Fulton D, Segal SS, Sessa WC. Temporal events underlying arterial remodeling after chronic flow reduction in mice: Correlation of structural changes with a deficit in basal nitric oxide synthesis. Circ Res. 2000;86:1160–1166 [DOI] [PubMed] [Google Scholar]
- 25.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: The “glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226 [DOI] [PubMed] [Google Scholar]
- 26.King AL, Polhemus DJ, Bhushan S, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A. 2014;111:3182–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altaany Z, Ju Y, Yang G, Wang R. The coordination of s-sulfhydration, s-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. 2014;7:ra87. [DOI] [PubMed] [Google Scholar]
- 28.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Guo Z, Wang S. The binding site for the transcription factor, nf-kappab, on the cystathionine gamma-lyase promoter is critical for lps-induced cystathionine gamma-lyase expression. International journal of molecular medicine. 2014;34:639–645 [DOI] [PubMed] [Google Scholar]
- 31.Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC. Laminar shear stress acts as a switch to regulate divergent functions of nf-kappab in endothelial cells. FASEB J. 2007;21:3553–3561 [DOI] [PubMed] [Google Scholar]
- 32.Mishanina TV, Libiad M, Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol. 2015;11:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ida T, Sawa T, Ihara H, et al. Reactive cysteine persulfides and s-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A. 2014;111:7606–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert review of clinical pharmacology. 2011;4:13–32 [DOI] [PubMed] [Google Scholar]
- 35.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific nf-kappab inhibition protects mice from atherosclerosis. Cell metabolism. 2008;8:372–383 [DOI] [PubMed] [Google Scholar]
- 36.Du J, Huang Y, Yan H, Zhang Q, Zhao M, Zhu M, Liu J, Chen SX, Bu D, Tang C, Jin H. Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-ldl)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor kappab (nf-kappab) pathway. J Biol Chem. 2014;289:9741–9753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of nf-kappab mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badiei A, Muniraj N, Chambers S, Bhatia M. Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity by downregulation of nf-kappab and map kinase activity in lps-activated raw 264.7 cells. BioMed research international. 2014;2014:848570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaddam RR, Fraser R, Badiei A, Chambers S, Cogger VC, Le Couteur DG, Ishii I, Bhatia M. Cystathionine-gamma-lyase gene deletion protects mice against inflammation and liver sieve injury following polymicrobial sepsis. PLoS One. 2016;11:e0160521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, Xu C, Wang R. Increased neointimal formation in cystathionine gamma-lyase deficient mice: Role of hydrogen sulfide in alpha5beta1-integrin and matrix metalloproteinase-2 expression in smooth muscle cells. J Mol Cell Cardiol. 2012;52:677–688 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179 [DOI] [PubMed] [Google Scholar]
- 42.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baeyens N, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell. 2016;27:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature. 1999;399:601–605 [DOI] [PubMed] [Google Scholar]
- 46.Huang B, Chen CT, Chen CS, Wang YM, Hsieh HJ, Wang DL. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun. 2015;464:1254–1259 [DOI] [PubMed] [Google Scholar]
- 47.Yokomizo A, Takatori S, Hashikawa-Hobara N, Goda M, Kawasaki H. Characterization of perivascular nerve distribution in rat mesenteric small arteries. Biological & pharmaceutical bulletin. 2015;38:1757–1764 [DOI] [PubMed] [Google Scholar]
- 48.Lidington D, Li F, Tyml K. Deletion of neuronal nos prevents impaired vasodilation in septic mouse skeletal muscle. Cardiovasc Res. 2007;74:151–158 [DOI] [PubMed] [Google Scholar]
- 49.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488 [DOI] [PubMed] [Google Scholar]
- 50.Kuhlencordt PJ, Hotten S, Schodel J, Rutzel S, Hu K, Widder J, Marx A, Huang PL, Ertl G. Atheroprotective effects of neuronal nitric oxide synthase in apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:1539–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schodel J, Padmapriya P, Marx A, Huang PL, Ertl G, Kuhlencordt PJ. Expression of neuronal nitric oxide synthase splice variants in atherosclerotic plaques of apoe knockout mice. Atherosclerosis. 2009;206:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolluru GK, Shen X, Kevil CG. A tale of two gases: No and h2s, foes or friends for life? Redox Biol. 2013;1:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortese-Krott MM, Kuhnle GG, Dyson A, et al. Key bioactive reaction products of the no/h2s interaction are s/n-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A. 2015;112:E4651–4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan S, Patel RP, Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. American journal of physiology. Lung cellular and molecular physiology. 2015;308:L403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.