Abstract
Non-myeloablative conditioning, such as with total lymphoid irradiation and anti-thymocyte globulin (TLI-ATG), has allowed hematopoietic allotransplantation with curative potential for older patients and those with comorbid medical conditions with myeloid neoplasms. However, early achievement of full donor chimerism (FDC) and relapse remain challenges. Cytokine induced killer (CIK) cells have been shown to have anti-tumor cytotoxicity. Infusion of donor-derived CIK cells has been studied for hematologic malignancies relapsed after allotransplant but has not been evaluated as post-transplant consolidation. In this phase II study, we prospectively studied whether a one-time infusion of 1 X 108/kg CD3+ donor-derived CIK cells administered between Days +21–35 after TLI-ATG conditioning, could improve FDC achievement by Day +90 and 2-year clinical outcomes in patients with myeloid neoplasms. CIK cells were infused in 31 of 44 patients treated on study and contained predominantly CD3+CD8+NKG2D+ cells along with significantly expanded CD3+CD56+ cells. Outcomes were compared to a retrospective historical cohort of 100 patients. We found that this one-time CIK infusion did not increase the rate of FDC by Day +90. On an intention-to-treat analysis, 2-year non-relapse mortality (6.8%, 95%CI: 0–14.5%), event-free survival (27.3%, 95%CI: 16.8–44.2%), and overall survival (50.6%, 95%CI: 37.5–68.2%) were similar to our historical cohort. Cumulative incidence of grade II-IV acute graft versus host disease at 1-year was 25.1% (95%CI: 12–38.2%). On univariate analysis, the presence of monosomal or complex karyotype was adversely associated with relapse-free and overall survival. Given the favorable safety profile of CIK cell infusion, strategies such as repeat dosing or genetic modification are worth exploration. This trial was registered at Clinicaltrials.gov ().
Keywords: CIK cells, NMA transplant, RIC transplant, MDS, AML, cell therapy
Graphical abstract
Introduction
Reduced intensity (RIC) and non-myeloablative (NMA) conditioning regimens are often utilized for patients with myeloid neoplasms of older age or with concurrent comorbid conditions undergoing allogeneic hematopoietic cell transplant (allo-HCT) due to high incidence of treatment related toxicity with myeloablative conditioning. In an analysis of 61 patients with myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN), or therapy-related myeloid neoplasms (t-MN) undergoing NMA conditioning combining total lymphoid irradiation (TLI) with anti-thymocyte globulin (ATG),1–3 3-year overall survival (OS) was 41% with 3-year non-relapse mortality (NRM) of 11%.2 Though low rates of NRM with curative potential are attractive features of TLI-ATG and other NMA conditioning regimens, early achievement of full donor chimerism (FDC), which has been associated with disease control,3–5 remains a challenge for patients with myeloid neoplasms.
We have shown that cytokine induced killer (CIK) cells can be derived ex-vivo from peripheral blood culture with interferon (IFN)-γ, interleukin (IL)-2, and anti-CD3.6,7 CIK cells harvested with this approach generally include populations of CD3+CD56+ as well as CD3+CD314/NKG2D+ (natural killer group 2, member D) cells.7,8 CD3+CD56+ cells have been shown to have non-MHC restricted anti-tumor cytotoxic activity.9 NKG2D, a member of the C-type lectin-like receptor family,10 has been associated with anti-tumor immune activity.11
CIK cells have been shown to have anti-tumor effects in-vitro, in severe combined immunodeficiency murine models with tumor, and in clinical studies.7,12 While the majority of CIK cell clinical studies have been in the autologous setting in hematologic neoplasms and solid tumors, a few studies have evaluated allogeneic donor-derived CIK cell infusion for hematological neoplasms relapsed after allo-HCT.8,13–15 In the first reported phase I allogeneic CIK cell infusion study,13 11 patients with hematologic disease relapse after allo-HCT received repeated infusions of CIK cells with a median of 12.4 X 106/kg total CIK cells per patient; in this study, 2 patients with mixed donor chimerism (MDC) subsequently converted to FDC, including 1 patient with CMML and 1 patient with MDS, both of whom experienced a complete response.13 In our previous phase I/II study of allogeneic CIK cells from matched related sibling donors in 18 patients with hematologic malignancies relapsed after allo-HCT, we evaluated dose escalations from 1 X 107/kg (n=4), 5 X 107/kg (n=6), to the highest planned dose of 1 X 108/kg CD3+ cells (n=8). We found that the CIK cells could be safely administered in the relapsed setting and had a low incidence of acute graft versus host disease (aGVHD).8
We hypothesized that early post-transplant consolidation with donor-derived CIK cells would be well-tolerated, have anti-tumor activity, and promote early donor chimerism, without significantly affecting rates of aGVHD. As post-transplant consolidation with allogeneic CIK cells has not been previously studied, we chose a dose of a one-time infusion of 1 X 108/kg CD3+ donor-derived CIK cells to be given between days +21–35 following TLI-ATG based allo-HCT for patients with MDS, MPN, t-MN and secondary acute myeloid leukemia (s-AML). Our primary objective was to determine the proportion of patients achieving FDC by Day +90 post-transplant. Our secondary objectives were to determine 2-year OS, 2-year event-free survival (EFS), aGVHD incidence, and to assess NKG2D ligand expression.
Materials and Methods
Study patients and donors
This was a single center, nonrandomized, open-label phase II trial (study protocol 217) evaluating the effects of a one-time infusion of donor-derived CIK cells following allo-HCT with TLI-ATG conditioning for patients with myeloid neoplasms. This study was approved by the Scientific Review Committee and Institutional Review Board of Stanford University, and the FDA (IND number 14307). It was performed in accordance with the principles of the Declaration of Helsinki. All subjects provided written informed consent and were treated at Stanford University Medical Center. Fifty-six patients provided informed consent; 12 patients were found to be ineligible and 44 patients were treated on study between 03/2011 through 02/2016. Data were censored using last follow-up visit before 08/2017 to allow minimal follow-up of 18 months.
Study eligibility
Eligibility criteria are further detailed in supplementary methods. Briefly, recipient eligible diagnoses included MDS; MPN excluding Philadelphia positive chronic myeloid leukemia (CML); MDS-MPN overlap syndromes; s-AML; and t-MN (including t-MDS, t-MPN, t-MDS-MPN overlap and t-AML). To be eligible, recipients had to be greater than 50 years of age or felt to be at high risk for toxicity from myeloablative conditioning, with availability of a fully HLA-matched or single antigen/allele mismatched related or unrelated donor. Recipient exclusion criteria included: uncontrolled central nervous system involvement; Karnofsky performance score (KPS) less than 70; documented progressive infections; viral hepatitis; HIV positivity; pregnant or lactating females; or evidence of organ dysfunction.
Donors were considered eligible if they: were age less than or equal to 75; had negative HIV and HBV nucleic acid testing within 30 days of collection; had adequate venous access for leukapheresis or be willing to undergo insertion of central venous catheter if needed; and willing to undergo a second donation of peripheral blood or bone marrow harvest for graft failure. Donor exclusion criteria included: identical twins for related donors; pregnant or lactating females; or for any medical, physical or psychological reasons that would increase the risk of complications from growth factor or leukapheresis.
Study treatment
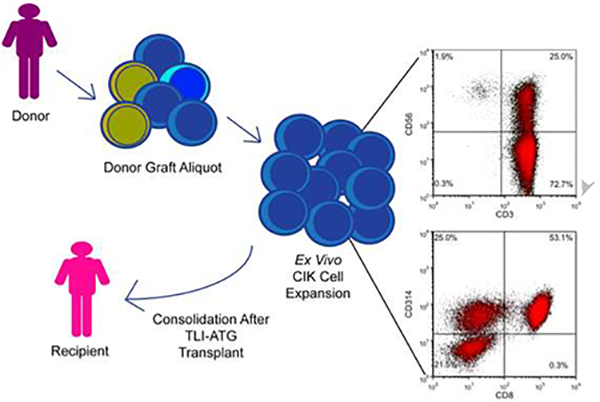
TLI was given as a total dose of 1200 cGy in 10 fractions (given on Days −11 to Day −7 and Day −4 to Day −1).1–3 Rabbit-derived ATG was given on days −11 to −7 at a daily dose of 1.5 mg/kg/day for a total dose of 7.5 mg/kg with solumedrol 1 mg/kg pre-medication prior to each dose. Peripheral blood hematopoietic progenitor cells were collected via G-SCF mobilization with apheresis on Day −1 and Day 0. While a CD34+ cell dose of greater than or equal to 5 X 106/kg of recipient body weight was targeted, transplants with less than target could still proceed. Additional apheresis was allowed for related donors if target CD34+ cells were not met. GVHD immunosuppression with cyclosporine (CSA) and mycophenolate mofetil (MMF) have been previously described.2 Standard institutional guidelines were followed for infection prophylaxis and treatment. An aliquot (less than 5%) of freshly collected primary graft was utilized for CIK cell expansion. CIK cell expansion method has been previously described7,8 (see supplementary methods). CIK cultures were not initiated when graft CD34+ cell dose was less than 5 X 106/kg and discontinued for recipient grade 2 or higher aGVHD or infection prior to scheduled infusion. CIK cells were harvested and infused between Days +21–35 at a target dose of 1 X 108 CD3+ cells per recipient kg. Cells were infused fresh without cryopreservation.
CIK cell immunophenotyping and in vitro cytotoxicity assays
Aliquots of harvested CIK cells were evaluated by flow cytometry for immunophenotype and for in vitro anti-tumor cytotoxicity against 4 common tumor cell lines (Jurkat, OCI-Ly8, SU-DHL4, and DB) using CIK effector to tumor target (E:T) cell ratios of 0:1, 10:1, 20:1, and 40:1. Methods have been described previously8 (see supplemental methods).
Laboratory assessments
Weekly blood counts, chemistries, and liver function tests were obtained post-transplant until Day +90 or more frequently as clinically indicated. Bone marrow aspirate/biopsies were obtained per institutional guidelines generally at 3, 6, and 12 months post-transplant and then yearly until year 5 post-transplant. Donor chimerism was assessed as previously described2,16 and performed no more than 48 hours prior to CIK infusion or by Day +28; and again around Day +56 and Day +90 +/−10 days, and subsequently annually or more frequently at clinical discretion. For the comparison historical cohort described below, donor chimerism data was retrospectively notated as available. FDC was defined as attainment of >95% peripheral donor-type CD3+ cells. MDC was defined as having between 5% and 95% peripheral donor-type CD3+ cells and graft failure (GF) was defined as having less than 5% donor-type CD3+ peripheral cells without concurrent relapse.
Historical cohort
For outcome comparison, we retrospectively evaluated data from 100 patients with similar diagnoses consecutively enrolled on TLI-ATG based NMA protocols (153, 9153, 168) between 04/2004 through 10/2015 (termed historical cohort). The conditioning regimen included TLI with a total cumulative dose of either 800 cGy or 1200 cGy in 10 fractions combined with ATG 1.5 mg/kg/day for 5 days. Donor CD34+ graft source and dose, along with immunosuppression taper (CsA/MMF) have been described1–3 and are similar to the current study.
Measurement of pre-transplant characteristics
IPSS17 and R-IPSS18 scores were assigned to patients with de novo MDS and t-MDS. ELN prognostic status19 was assigned for patients with AML. Types of pre-transplant therapy, presence of known prior monosomal20 or complex karyotype,19,21 and HCT-CI score22 were notated. Pre-transplant disease status was assessed using the International Working Group (IWG) criteria for MDS and ELN criteria for AML,19,23 but with persistence of myeloid neoplasm morphologically notated as persistent disease for secondary AML for statistical analyses. Grouping of pre-transplant disease status and types of therapy received prior to transplant for statistical analyses are detailed in supplementary methods.
Statistical analyses
The primary objective of the study was to determine the proportion of patients achieving FDC on or by Day +90 after infusion of allogeneic CIK cells following alloHCT with TLI-ATG conditioning. Secondary objectives included determination of 2-year OS rate, 2-year EFS rate, incidence of aGVHD, and assessment of NKG2D ligand expression. The study was designed as a Simon-2 stage optimal phase II single arm study with enrollment target of 21 patients, using a 10% type I error and 80% power to detect an improvement of FDC from 25% (based on historical rate) to 50% on/by Day +90; enrollment was increased to 44, as our initial goals did not clearly distinguish between phase I dose finding for unrelated donors (n=4) and for patients who would not receive CIK cells due to low graft cell count, other illness, or logistical reasons.
For determination of the primary endpoint, patients achieving FDC on or by approximately Day +90 among those receiving CIK cell infusion were counted, including those who achieved FDC but died before Day +90; in cases where chimerism data availability were limited, the closest values before Day 118 were utilized. Post-transplant persistent disease (including >/= 10% morphologic dysplasia for MDS; persistence of any disease related cytogenetic abnormalities), disease relapse (including reappearance of circulating blasts, new extramedullary disease, re-occurrence or evolution of disease related cytogenetic abnormalities) and disease progression were grouped together as relapsed disease. The secondary endpoints of OS, EFS, and aGVHD incidence were analyzed on all enrolled patients. The OS rate was analyzed using death from any cause using Kaplan Meier (KM) estimator from the day of transplant with censoring time being the date of last follow-up or date of second transplant. Median follow-up was estimated based on reverse KM estimator.24 In analyzing EFS rate, events were defined as relapse, grade III-IV aGVHD, or death.
RFS was defined using relapse or death as events. Cumulative incidence of relapse was calculated in the competing risk model including GF or death as competing risks. Cumulative incidence of GF was calculated treating relapse or death as competing risks. Cumulative incidence of NRM was calculated treating relapse as a competing risk. OS, EFS, and RFS were compared between the study and historical cohorts using the log-rank method. Acute GVHD was graded using the modified Keystone criteria.25 Cumulative incidence of aGVHD grade II-IV was calculated treating aGVHD max grade I, GF, or death as competing risks. Chronic GVHD (cGVHD) was graded using the NIH scale.26 Cumulative incidence of cGVHD was calculated using GF or death as competing risks. For comparison of characteristics between the study and historical cohorts, Fisher’s exact test and the Mann-Whitney test were used for categorical and continuous variables respectively. Univariate analyses for RFS and OS were completed using the cox proportional hazards method. Univariate analyses for CIK cell subset versus outcome was completed using logistic regression method. The statistical significance level for all tests was set at 0.05. Statistical analyses were conducted using R3.2.2 (The R foundation for statistical computing).
Results
Patient baseline characteristics
The median age of the study cohort was 64.5 years (range, 37–74) with 48% female and 52% male patients, with most patients having a baseline KPS of 80–90. Baseline characteristics are summarized in Table 1. Disease diagnoses included MDS (61%), s- or t-AML (27%), MPN (5%), and MDS/MPN overlap syndromes (7%). Of patients with MDS, 55% had RAEB-1 or RAEB-2 type disease, and 15% had t-MDS. All t-MDS patients had IPSS higher risk disease (Int-2 or High). Of the 23 patients with de novo MDS, 48% had higher risk disease by IPSS. Complex or monosomal karyotypes at disease diagnosis were noted in 21% and 16% of patients respectively. In terms of therapy prior to transplant, 66% of patients had therapy with hypomethylating agents (HMAs) and/or immunomodulators (IMIDs); 14% had cytoreductive induction-type chemotherapy regimens; and 14% had both prior cytoreductive therapy and prior HMAs and/or IMIDs. The majority of AML patients (8 of 12, 67%) were in CR at the time of transplant. For patients with MDS, 33% were in a CR, 41% had a response less than CR, and 26% were either non-responsive to prior therapy or had progressive disease at the time of transplant.
Table 1.
Demographics and Disease Characteristics
| Variable | Historical Cohort (n=100) | Study 217 Cohort (n=44) | p-value |
|---|---|---|---|
| Median age, years (range) | 63 (46–74) | 64.5 (37–74) | P=0.1688 |
| Age, years | P=0.3202 | ||
| <60 | 30 (30%) | 9 (20%) | |
| Year of HCT | P=3.303e-13 | ||
| 2001–2010 | 58 (58%) | 0 (0%) | |
| Sex, Male/Female | 55 (55%) / 45 (45%) | 23 (52%) / 21 (48%) | P=0.8563 |
| Pre-transplant KPS | P=0.02773 | ||
| 70 | 3 (3%) | 0 (0%) | |
| HCT-CI Score | P=0.07767 | ||
| 0 | 60 (60%) | 18 (41%) | |
| Disease Type | |||
| AML | 33 (33%) | 12 (27%) | P=1 |
| Secondary / t-AML | 20 (61%) / 13 (39%) | 8 (67%) / 4 (33%) | |
| MDS | 52 (52%) | 27 (61%) | P=0.1537 |
| RARS, RARS-T, or RCMD | 16 (31%) | 8 (30%) | |
| MPN | 7 (7%) | 2 (5%) | P=1 |
| De novo / t-MPN | 6 (86%) / 1 (14%) | 2 (100%) / 0 (0%) | |
| MDS/MPN Overlap | 8 (8%) | 3 (7%) | P=0.05455 |
| De novo / t-MDS/MPN overlap | 8 (100%) / 0 (0%) | 1 (33%) / 2 (66%) | |
| IPSS Risk Classification, of de novo MDS | 36 | 23 | P=0.3105 |
| Low or lnt-1 | 18 (50%) | 11 (48%) | |
| IPSS Risk Classification, of t-MDS | 16 | 4 | P=1 |
| Low or lnt-1 | 2 (12%) | 0 (0%) | |
| R-IPSS Risk Classification, of de novo MDS | 36 | 23 | P=0.4513 |
| Very Low or Low | 7 (19%) | 3 (13%) | |
| R-IPSS Risk Classification, of t-MDS | 16 | 4 | P=1 |
| Very Low or Low | 1 (6%) | 0 (0%) | |
| ELN Classification for AML | 33 | 12 | P=0.5275 |
| Favorable | 3 (10%) | 1 (8%) | |
| Complex Cytogenetics | P=0.8648 | ||
| No / Yes / Unknown | 63 (63%) / 22 (22%) / 15 (15%) | 30 (68%) / 9 (21%) / 5 (11%) | |
| Monosomal Karyotype | P=0.8218 | ||
| No / Yes / Unknown | 67 (67%) / 18 (18%) / 15 (15%) | 32 (73%) / 7 (16%) / 5 (11%) | |
| Therapies prior to transplant | P=0.03261 | ||
| Cytoreductive induction type therapy only | 29 (29%) | 6 (14%) | |
| Of prior HMA, type of HMA | 45 | 32 | P=0.1377 |
| Decitabine alone | 9 (20%) | 9 (28%) | |
| Disease Status at Transplant, AML | 33 | 12 | P=0.3366 |
| Morphological CR | 20 (61%) | 8 (68%) | |
| Disease Status at Transplant, MDS | 52 | 27 | P=0.2975 |
| CR | 10 (19%) | 9 (33%) | |
| Disease status at Transplant, MPN or MDS/MPN Overlap | 15 | 5 | P=0.3661 |
| CR | 3 (20%) | 0 (0%) | |
| Median time, diagnosis to HCT, Days (Range) | 247.5 (92–5502) | 227.5 (97–2342) | P=0.5401 |
Transplant characteristics
Transplant characteristics are summarized in Table 2. Sixty-four percent of donors were unrelated. Thirty-nine percent of patients received gender mismatched transplants. There was CMV donor/recipient seropositivity discordance of 30% (reactive/nonreactive or nonreactive/reactive). The median graft CD34+ dose was 7.1 X 106/kg (range 2.3–17). Of the 44 patients treated on study, 31 received CIK cells at a median time of 26 days post-allogeneic stem cell infusion (range 24–31 days; termed CIK recipient subgroup). CIK recipient subgroup characteristics and outcomes are detailed in supplementary tables. CIK cells were infused in the ambulatory setting with no major infusion reactions noted. As part of our phase I dose finding assessment for patients with unrelated donors, the CIK CD3+ cell dose was escalated from 1 X 107/kg (n=1) to 3 X 107/kg (n=1) to 1 X 108/kg (n=2), amongst the first 4 patients with unrelated donors. Two other patients later received less than target CIK CD3+ 1 X 108 cells/kg (range 9.0 X 107/kg - 9.5 X 107/kg). Thirteen patients did not receive CIK cells due to the following reasons: 5 donor grafts had CD34+ cell dose below threshold for CIK culture initiation; 4 patients had aGVHD prior to scheduled infusion; 1 patient had fever with concern for active infection; and there were logistical issues with initiating CIK cells for 3 patients (including transplant delay and reagent unavailability).
Table 2.
Transplant Characteristics
| Main Category | Historical Cohort (n=100) | Study 217 cohort (n=44) | P-value |
|---|---|---|---|
| Donor Median Age (range) | 47(18–75) | 31.5 (21–72) | P=0.1683 |
| Donor Type | P=0.5549 | ||
| Matched Related | 39 (39%) | 16 (36%) | |
| Gender Donor/Recipient | P=0.4129 | ||
| F->F | 25 (25%) | 11 (25%) | |
| CMV Donor/Recipient | P=0.142 | ||
| Reactive/Reactive | 31 (31%) | 18 (41%) | |
| Median Graft CD34+ dose X 106 / kg (range) | 6.1 (1.8–21.9) | 7.1 (2.3–17) | P=0.0644 |
| Median Graft CD3+ dose X 108 / kg (range) | 2.9 (0.55–6.35) | 2.62 (1.44–6.87) | P=0.3256 |
For comparison of study outcomes, we evaluated retrospective data obtained from a historical cohort of 100 patients consecutively treated with TLI-ATG based allo-HCT for similar diagnoses. Baseline characteristics were mostly similar (Table 1); however, KPS and the type of therapy received prior to transplant differed between the two groups. A higher number of patients in the historical cohort had received prior cytoreductive induction-type chemotherapy and other care/supportive care. The median follow-up time by reverse KM estimator was longer in the historical cohort compared to the study cohort (2539 versus 1375 days, respectively).
CIK cell Immunophenotype by flow cytometry and in vitro cytotoxicity assays
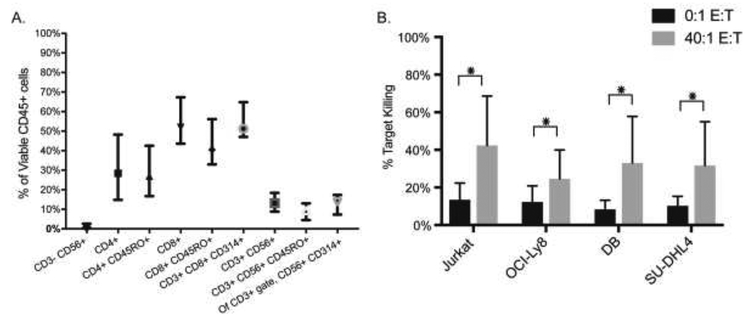
The majority of CIK cells were CD3+ (median 97.3%, range 59–99.8%) (Figure 1A, Supplemental Table 1). Of viable CD45+ cells, CD3+CD8+NKG2D/CD314+ were the predominant cell type (median 51.2%, range 8.1–80.9%), with the majority of CD8+ cells being of CD45RO+ memory phenotype (Figure 1A). Comparing graft seed aliquot to final CIK cell culture, the median percentile fold expansion of CD3+CD56+ cells was 15.3 (Supplemental Table S1). Supplemental Figure 1 shows sample flow plots comparing the graft seed aliquot and final CIK cell culture for one representative patient. Cytotoxic capability of CIK cells from 22 cultures were tested in vitro against 4 common tumor cell lines (Figure 1B). The mean tumor target cell killing at a ratio of 40:1 effectors to targets after a one-hour coincubation at 37°C was as follows: 42.3% (Jurkat, range 10.6–96.6%), 24.6% (OCI-Ly8, range 6.9–52.4%), 33.0% (DB, range 6.8–91.4%), and 31.7% (SU-DHL4, range 8.3–80.4%).
Figure 1. Characteristics of harvested CIK cells.
(A) Immunophenotype of CIK cells by flow cytometry (median and 95% CI are shown). (B) CIK cells from 22 patients were tested for in vitro cytotoxicity against 4 different tumor cell lines (Jurkat, OCI-Ly8, DB, and SU-DHL4) at varying effector CIK to tumor target (E:T) cell ratios. The percent of tumor target cell killing with 0:1 (no CIK cells) and 40:1 E:T (after one hour CIK co-incubation) is shown (mean with SD; *indicates p<0.05).
Transplant outcomes
Transplant outcomes are summarized in Table 3. For our primary endpoint, 6 out of 31 CIK recipients (19%) achieved FDC by Day +90, which was not significantly different from the overall study cohort (25%, p=0.780) or the historical cohort (27%, p=0.482), not meeting our target improvement to 50% with this one-time infusion intervention.
Table 3.
Transplant Outcomes
| Characteristic | Historical Cohort (n=100) | Study 217 Cohort (n=44) | |
|---|---|---|---|
| On/By Day +90 Chimerism | P=0.8194 | ||
| NA | 1 (1%) | 0 (0%) | |
| FDC Yes or No by/before Day +90 | P=0.8406 | ||
| Yes | 27 (27%) | 11 (25%) | |
| Graft Failure (GF) | P=0.5858 | ||
| No / Yes | 98/2 | 42/2 | |
| Relapse | |||
| Median time to Relapse, Days | 89 (13–3290) | 88 (25–600) | |
| Relapse-Free Survival (RFS) | |||
| 2-year RFS, % (95% Cl) | 27.9% (20.4–38.3%) | 27.3% (16.8–44.2%) | |
| Non-relapse mortality (NRM) | |||
| 2-year NRM, % (95% Cl) | 6.0% (1.3–10.7%) | 6.8% (0–14.5%) | |
| Event-Free Survival (EFS) | |||
| 2-year EFS, % (95% Cl) | 27.9% (20.4–38.3%) | 27.3% (16.8–44.2%) | |
| Overall Survival (OS) | |||
| Median OS, Days | 521 (353–1036) | 689 (373-) |
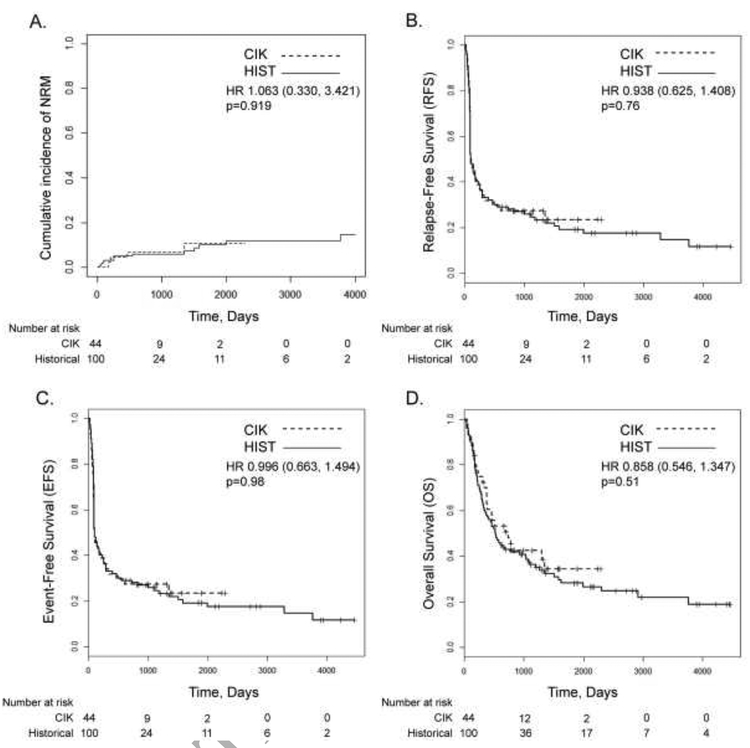
In terms of other clinical outcomes (Figure 2), the 2-year RFS was similar between the study and historical cohorts (27.3% (CI 16.8–44.2) versus 27.9% (CI 20.4–38.3); hazard ratio (HR) 0.938 [0.625, 1.408], p=0.76). Two-year EFS was also similar between the study and historical cohorts (27.3% (CI 16.8–44.2) versus 27.9% (CI 20.4–38.3); HR 0.996 [0.663,1.494], p=0.98). Two-year NRM was low and not significantly different between the study and historical cohorts (6.8% (CI 0–14.5) versus 6.0% (CI 1.3–10.7); HR 1.063 [0.330–3.421], p=0.919). While the median OS for the study cohort (689 days, CI 373-not reached) and CIK recipient subgroup (736 days, CI 373-not reached), trended higher than the historical cohort (521 days, CI 353–1036), the secondary endpoint of 2-year OS was not statistically significant (study cohort: 50.6% (CI 37.5–68.2) versus historical cohort: 42.9% (CI 34.2–53.8); HR 0.858 [0.546,1.347], p=0.51). Two-year OS for the CIK recipient subgroup was 52.6% (CI 37.3–74.2), which was not statistically significant compared to the historical cohort (p=0.36). As pre-transplant recipient tumor samples were generally not available, we were unable to assess NKG2D ligand expression as part of our secondary objectives.
Figure 2. Transplant Outcomes.
Cumulative incidence of NRM (A), RFS (B), EFS (C), and OS (D), are shown.
The 100-day cumulative incidence of grade II-IV aGVHD in the study cohort was 20.5% (CI 8.4–32.5) versus 12.0% for the historical cohort (CI 5.6–18.4, p=0.244) with 1-year grade II-IV aGVHD cumulative incidence of 25.1% (CI 12.0–38.2) for the study cohort versus 16.0% (CI 8.8–23.2, p=0.242) for the historical cohort (Table 4). In the CIK recipient subgroup, 100-day and 1-year cumulative incidence of grade II-IV aGVHD was 9.7% (CI 0–20.3) and 16.3% (CI 2.9–29.7). In the study cohort, 6 of 44 patients experienced grade III (5 patients) or IV (1 patient) aGVHD within the first year with 4 of these occurring within the first 100 days. While there was a statistical difference for 100-day and 1-year aGVHD grade III-IV between the study and historical cohorts (Table 4), given the small sample size and number of events, the p value and confidence interval need to be interpreted with caution. Two-year cumulative incidence of cGVHD for the study cohort (28.2%, CI 14.4–42.1) was similar to the historical cohort (30.2%, CI 21–39.3, p=0.829).
Table 4.
GVHD Outcomes
| Characteristic | Historical Cohort (n=100) | CIK Study Cohort (n=44) | CIK Recipients Subgroup (n=31) | p values |
|---|---|---|---|---|
| aGVHD | ||||
| Cumulative Incidence of aGVHD II-IV, % (95% Cl) | 100-day: 12.0% (5.6–18.4%) 1-year: 16.0% (8.8–23.2%) | 100-day: 20.5% (8.4–32.5%) 1-year 25.1% (12.0–38.2%) | 100-day: 9.7% (0–20.3%) 1-year: 16.3% (2.9–29.7%) | Study versus Historical 100-day: 0.244 1-year: 0.242 |
| Cumulative Incidence of aGVHD III-IV, % (95% CI)^ | 100-day: 1.0% (0–3.0%) 1-year: 2.0% (0–4.8%) | 100-day: 9.1% (0.5–17.7%) 1-year: 13.7% (3.3–24.2%) | 100-day: 3.2% (0–9.6%) 1-year: 9.9% (0–20.8%) | Study versus Historical 100-day: <0.001 1-year: <0.001 |
| cGVHD | ||||
| Cumulative Incidence of cGVHD, % (95% Cl) | 1-year: 26.0% (17.3–34.7%) 2-year: 30.2% (21.0–39.3%) 3-year: 32.6% (23.2–42.0%) | 1-year: 25.8% (12.4–39.2%) 2-year: 28.2% (14.4–42.1%) 3-year: NA | 1-year: 30.5% (13.4–47.7%) 2-year: 34.1% (16.3–51.9%) 3-year: NA | Study versus Historical 1-year: 0.982 2-year: 0.829 |
For comparison of aGVHD III-IV, given small sample size and number of events, results including p-value and confidence intervals need to be interpreted with caution.
Statistical analysis
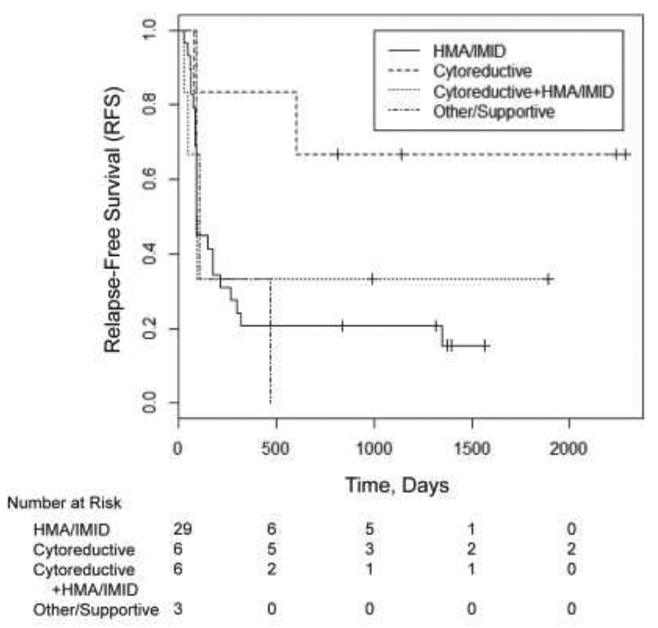
In univariate logistic regression analyses evaluating the percentile of CIK cell subsets in harvested cells (CD3+, CD3+CD56+, CD3+CD8+CD314+, CD8+, CD8+CD45RO+, CD4+,CD4+CD45RO+, CD3−CD56+ and CD3+CD56+CD314+), while there was no association of cell subsets with Day 90 chimerism, the percentile of CD3+CD56+ and CD3+CD56+CD314+ was significantly associated with increased odds of achieving post-transplant remission (CR or CRi) at Day 90 (see supplemental data). We also evaluated the impact of several pre-transplant variables on RFS and OS. On univariate analysis for the study cohort (Table 5), prior therapy with cytoreductive induction-type chemotherapy as compared to HMAs and/or IMIDs was associated with increased RFS (HR 0.233, p=0.049) (Figure 3). Presence of monosomal or complex karyotype was associated with an increased risk of relapse or death (HR 2.846 for RFS, p=0.00884). Having MPN or MPN/MDS overlap compared to MDS also appeared to be less favorable for RFS (HR 2.971, p=0.044). However, the only univariate that appeared to significantly affect OS for the study cohort was the presence of monosomal or complex karyotype (HR 3.075, p=0.009). Multivariate analysis using disease diagnosis, type of therapy prior to transplant, disease status at remission, presence of de novo versus secondary/therapy related myeloid neoplasm, cytogenetics at disease diagnosis (monosomal and/or complex versus not), HCT-CI score, donor to recipient gender mismatch, and age did not identify factors that were significantly associated with OS.
Table 5.
Study 217 Cohort: Univariate Analysis for RFS and OS (n=44)
| RFS | OS | |||||
|---|---|---|---|---|---|---|
| Therapy Prior to Transplant | ||||||
| HMA/IMID (reference) | 1.00 | 1.00 | ||||
| Disease Diagnosis | ||||||
| MDS (reference) | 1.00 | 1.00 | ||||
| Disease Remission Status at Transplant | ||||||
| CR (reference) | 1.00 | 1.00 | ||||
| Disease Cytogenetics at Diagnosis | ||||||
| Non-Monosomal/Non-Complex (reference) | 1.00 | 1.00 | ||||
| HCT-CI Score | ||||||
| Score 0 (reference) | 1.00 | 1.00 | ||||
| Donor:Recipient Gender Mismatch | ||||||
| No Mismatch (F:F or M:M) (reference) | 1.00 | 1.00 | ||||
| Age | ||||||
| 1 year older | 1.015 | 0.964–1.07 | 0.567 | 1.02 | 0.951–1.094 | 0.582 |
| Disease Type | ||||||
| Secondary or Therapy related (reference) | 1.00 | 1.00 |
Figure 3. Impact of therapy prior to transplant on RFS.
The effect of the type of therapy prior to transplantation on RFS was assessed.
Discussion
In our prior experience with TLI-ATG conditioning for MDS and MPN, we found a 6-month median time to FDC post-transplant, with decreased relapse rates in patients achieving early donor reconstitution.2 We evaluated a one-time infusion of CIK cells as post-transplant consolidation to determine whether this intervention could promote early FDC achievement and potentially decrease relapse rates. Our study population included a relatively high-risk population including 80% of patients being age 60 and above; 27% with HCT-CI scores of 3 or greater; and 23% having t-MN, with the majority of MDS patients having intermediate or higher risk disease by R-IPSS. Sixty-one percent of patients had either a response less than CR or persistent/progressive disease at the time of transplant.
We found that CIK cell infusion was safe and feasible to infuse in the ambulatory setting as early consolidation therapy, with similar rates of 100-day and 1-year grade II-IV aGVHD. One limitation of our study was the non-randomized design. Thus, study results were compared to retrospective historical data. However, the study and historical groups were broadly similar at baseline, with the back-bone of TLI-ATG conditioning for similar diagnoses of myeloid neoplasms using the same GVHD prophylaxis, which facilitated an overall comparison of outcome evaluation. The rate of FDC by Day +90 following CIK infusion in our study population was not increased compared to the historical cohort. Cumulative incidence of relapse was similar between the study and historical cohorts, with relapse being the main cause of treatment failure and the majority of relapse events occurring within the first-year post transplant, similar to what has been described for AML and MDS patients.27,28 We observed a low rate of 1- and 2-year NRM (<10%), which compares favorably to recent 3-year transplant related mortality data reported from CIBMTR for allo- HCT for AML (20–22%) and MDS (26–37%) collected between 1998–2011.27
Two-year OS data in this study was also generally similar to that previously reported for MDS and AML.27–29 While the median OS trended higher in our study cohort compared to historical cohort (689 days versus 521 days), the difference in 2-year OS was not statistically significant. Further, a significant proportion of patients in the study group did not receive the planned CIK cell infusion (13 of 44) for a variety of reasons. For this reason, we analyzed the subgroup of patients who did receive CIK cells and while these patients had a trend for improved OS, relapse rates were not reduced.
The reasons for failure to see improved outcomes are potentially complex. While prior work suggested that immunosuppressive medications such as CsA predominantly affected anti-CD3 based degranulation but not cytotoxicity,30 it is possible that CsA or MMF may have impacted CIK cell function, proliferation, or that CIK cells did not survive for a sufficiently long enough time period. While effects of residual ATG on CIK cells are a possibility, our prior finding of residual ATG not being detected in recipient serum one week after TLI-ATG conditioning3 makes this less likely. The expression of exhaustion markers on healthy-donor derived CIK cells after infusion has not been well-studied. One study of checkpoint expression in autologous CIK cells derived from lung cancer patients,31 found that the expression of several markers, such as LAG3 and PD-L1, became elevated during early culture points and remained elevated above baseline over the course of a 21 day culture; whereas expression of others such as PD-1 and CTLA-4 returned towards baseline at the end of culture. As CIK cells in our study were not modified with a marker, we were unable to follow their survival and evaluate checkpoint expression post-infusion but monitoring such expression may be helpful in the future. A one-time infusion of CIK cells as may also have been insufficient against the clonal burden present. Repeat dosing strategies have been evaluated for disease relapsed after allo-HCT.13 Given the overall safety of CIK infusion as early post-transplant consolidation that we found in this study, repeat dosing schedules may be considered at this time point as well.
Other strategies to enhance CIK cell efficacy are also worth exploration. Several groups have evaluated substitution of the use of IL-2 during CIK culture to other cytokines, such as IL15,32 as a potential way to increase CIK cell cytotoxicity and downregulate T-regulatory cells within the CIK culture. CIK cells modified with chimeric antigen receptors (CARs) are being developed against both lymphoid and myeloid targets.33–35 CIK cells modified using an oncolytic virus interestingly showed tumor regression in mouse models.36 Strategies enhancing anti-tumor specific targeting without increasing NRM and GVHD are likely to improve overall clinical outcomes.
In univariate analysis evaluating the effect of pre-transplant variables on RFS and OS, type of prior therapy, presence of monosomal or complex karyotype, and disease type were found to impact RFS, with monosomal or complex karyotype impacting OS. In our multivariate analysis we did not find any variables with significant association to OS. Monosomal karyotype has been found to be associated with poor survival in MDS and AML patients in several studies.27,37–40 The impact of pre-transplant therapy type in patients with MDS on post-transplant outcomes has been mixed,41–46 and may be confounded by age, fitness at diagnosis, and cytogenetic/molecular features. Current consensus guidelines47 currently recommend consideration of pre-transplant therapy for higher risk MDS patients with more than 10% blasts but without a recommendation for optimal type of pre-transplant disease modifying therapy.47 Intriguingly, in a prospective trial evaluating hypomethylation therapy with azacitidine for early post-transplant relapse in AML and MDS,48 pre-transplant intensified chemotherapy was associated with increased response to salvage azacitidine and improved OS on multivariate analysis. How pre-transplant induction type chemotherapy affects post-transplant outcomes specifically in non-chemotherapy based NMA conditioning regimens will be important to better understand as NMA regimens are increasingly being used for older patients with AML and MDS.
In summary, in this relatively high-risk population, we confirmed that TLI-ATG based conditioning has a relatively low risk of NRM with survival benefit in patients with myeloid neoplasms. While we did not see earlier FDC or improved relapse rates with a one-time CIK cell infusion following TLI-ATG conditioning, we found that such infusion was overall safe as post-transplant consolidation. Varied dosing strategies or CIK cell modification may enhance the anti-tumor efficacy of this treatment modality.
Supplementary Material
Highlights.
Phase II study of CIK cell therapy after TLI-ATG conditioning for myeloid neoplasms
Donor-derived CIK cells were produced from both related and unrelated donors
A one-time CIK cell infusion as early post-transplant consolidation was safe
Early donor chimerism and relapse/survival rates were similar to historical rates
Acknowledgements
We thank the staff of the Stanford BMT Cell Therapy Facility for performing CIK cell culture manufacture. We also thank Linda Elder, along with the rest of the Stanford BMT Database team for assistance with data collection. This work was supported by the National Institutes of Health (PPG CA49605). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: The authors declare no competing financial interests.
Presented in abstract form at the 55th and 57th annual meeting of the American Society of Hematology, Orlando, FL
References
- 1.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114(5):1099–1109. doi: 10.1182/blood-2009-03-211441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin J, Chhabra S, Kohrt HE, et al. Total lymphoid irradiation-antithymocyte globulin conditioning and allogeneic transplantation for patients with myelodysplastic syndromes and myeloproliferative neoplasms. Biol Blood Marrow Transplant. 2014;20(6):837–843. doi: 10.1016/j.bbmt.2014.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–1331. doi: 10.1056/NEJMoa050642 [DOI] [PubMed] [Google Scholar]
- 4.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94(9):3234–3241. [PubMed] [Google Scholar]
- 5.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104(8):2254–2262. doi: 10.1182/blood-2004-04-1506 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153(4):1687–1696. [PubMed] [Google Scholar]
- 8.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1679–1687. doi: 10.1016/j.bbmt.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA. Comparative studies of CD3- and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol. 1991;136(2):486– 495. [DOI] [PubMed] [Google Scholar]
- 10.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27(45):5944–5958. doi: 10.1038/onc.2008.272 [DOI] [PubMed] [Google Scholar]
- 11.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580. doi: 10.1016/j.immuni.2008.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Introna M, Correnti F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int J Mol Sci. 2018;19(2). doi: 10.3390/ijms19020358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. doi: 10.3324/haematol.11132 [DOI] [PubMed] [Google Scholar]
- 14.Linn YC, Niam M, Chu S, et al. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012;47(7):957–966. doi: 10.1038/bmt.2011.202 [DOI] [PubMed] [Google Scholar]
- 15.Introna M, Lussana F, Algarotti A, et al. Phase II Study of Sequential Infusion of Donor Lymphocyte Infusion and Cytokine-Induced Killer Cells for Patients Relapsed after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(12):2070–2078. doi: 10.1016/j.bbmt.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73(9):1386–1391. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 18.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Döhner H, Estey EHE, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 20.Breems DA, Van Putten WLJ, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. doi: 10.1200/JCO.2008.16.0259 [DOI] [PubMed] [Google Scholar]
- 21.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820–829. doi: 10.1200/JCO.2011.35.6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi: 10.1182/blood-2005-10-4149 [DOI] [PubMed] [Google Scholar]
- 24.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. [DOI] [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 26.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Pasquini MC, Zhang MJ, Medeiros BC, et al. Hematopoietic Cell Transplantation Outcomes in Monosomal Karyotype Myeloid Malignancies. Biol Blood Marrow Transplant. 2016;22(2):248–257. doi: 10.1016/j.bbmt.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saber W, Cutler CS, Nakamura R, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood. 2013;122(11):1974–1982. doi: 10.1182/blood-2013-04-496778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robin M, Ruggeri A, Labopin M, et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunob. Biol Blood Marrow Transplant. 2015;21(3):489–495. doi: 10.1016/j.bbmt.2014.11.675 [DOI] [PubMed] [Google Scholar]
- 30.Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86(9):3493–3499. [PubMed] [Google Scholar]
- 31.Zhang L, Wang J, Wei F, et al. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients. Oncotarget. 2016;7(28):43604–43615. doi: 10.18632/oncotarget.9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Q, Chen T, Tao L, et al. IL-15 improves the cytotoxicity of cytokine-induced killer cells against leukemia cells by upregulating CD3+CD56+ cells and downregulating regulatory T cells as well as IL-35. J Immunother. 2013;36(9):462–467. doi: 10.1097/CJI.0000000000000001 [DOI] [PubMed] [Google Scholar]
- 33.Rotiroti MC, Arcangeli S, Casucci M, et al. Acute Myeloid Leukemia Targeting by Chimeric Antigen Receptor T Cells: Bridging the Gap from Preclinical Modeling to Human Studies. Hum Gene Ther. 2016;28(3):231–241. doi: 10.1089/hum.2016.092 [DOI] [PubMed] [Google Scholar]
- 34.Tettamanti S, Marin V, Pizzitola I, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389–401. doi: 10.1111/bjh.12282 [DOI] [PubMed] [Google Scholar]
- 35.Biondi A, Magnani CF, Tettamanti S, Gaipa G, Biagi E. Redirecting T cells with Chimeric Antigen Receptor (CAR) for the treatment of childhood acute lymphoblastic leukemia. J Autoimmun. 2017;85:141–152. doi: 10.1016/j.jaut.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 36.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311(5768):1780–1784. doi: 10.1126/science.1121411 [DOI] [PubMed] [Google Scholar]
- 37.Della Porta MG, Alessandrino EP, Bacigalupo A, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123(15):2333–2342. doi: 10.1182/blood-2013-12-542720 [DOI] [PubMed] [Google Scholar]
- 38.Pourhassan H, Defor T, Trottier B, et al. MDS disease characteristics, not donor source, predict hematopoietic stem cell transplant outcomes. Bone Marrow Transplant. 2017;52(4):532–538. doi: 10.1038/bmt.2016.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Gelder M, de Wreede LC, Schetelig J, et al. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2013;27(4):879–888. doi: 10.1038/leu.2012.297 [DOI] [PubMed] [Google Scholar]
- 40.Koenecke C, Gohring G, de Wreede LC, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic synd. Haematologica. 2015;100(3):400–408. doi: 10.3324/haematol.2014.116715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakai K, Kanda Y, Fukuhara S, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19(3):396–401. doi: 10.1038/sj.leu.2403640 [DOI] [PubMed] [Google Scholar]
- 42.Scott BL, Storer B, Loken MR, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11(1):65–73. doi: 10.1016/j.bbmt.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 43.de Witte T, Hagemeijer A, Suciu S, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European intergroup trial. Haematologica. 2010;95(10):1754–1761. doi: 10.3324/haematol.2009.019182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onida F, Brand R, van Biezen A, et al. Impact of the International Prognostic Scoring System cytogenetic risk groups on the outcome of patients with primary myelodysplastic syndromes undergoing allogeneic stem cell transplantation from human leukocyte antigen-identical siblings: a retrospecti. Haematologica. 2014;99(10):1582–1590. doi: 10.3324/haematol.2014.106880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant. 2012;18(8):1211–1218. doi: 10.1016/j.bbmt.2012.01.009 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damaj G, Duhamel A, Robin M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol. 2012;30(36):4533–4540. doi: 10.1200/JCO.2012.44.3499 [DOI] [PubMed] [Google Scholar]
- 47.Witte T De, Bowen D, Robin M, et al. Review Article Allogeneic hematopoietic stem cell transplantation for MDS and CMML : recommendations from an international expert panel. Blood. 2017;129(13):1753–1763. doi: 10.1182/blood-2016-06-724500.BLOOD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo J, Deeg HJ, Storer B, et al. Factors Determining Responses to Azacitidine in Patients with Myelodysplastic Syndromes and Acute Myeloid Leukemia with Early Post-Transplantation Relapse: A Prospective Trial.Biol Blood Marrow Transplant. 2017;23(1):176–179. doi: 10.1016/j.bbmt.2016.10.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.