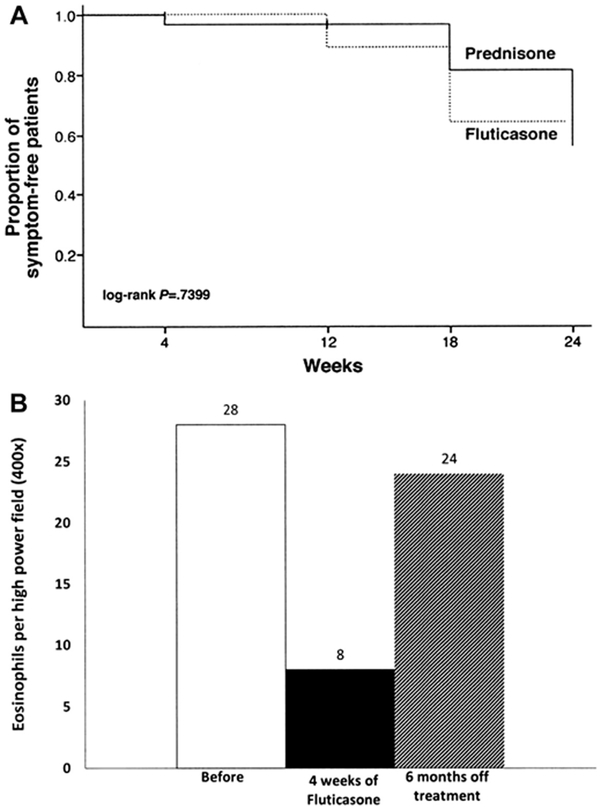
Fig. 1.
(A) Proportion of symptom-free patients with prednisone and swallowed fluticasone. Patients received induction dose × 4 weeks, were weaned over 8 weeks, and were clinically monitored for next 12 weeks. (B) Recurrence of esophageal eosinophilia after withdrawal of swallowed fluticasone (220 mg twice daily). (From [A] Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol 2008;6:165–73, with permission; and [B] Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005;3:1202, with permission.)