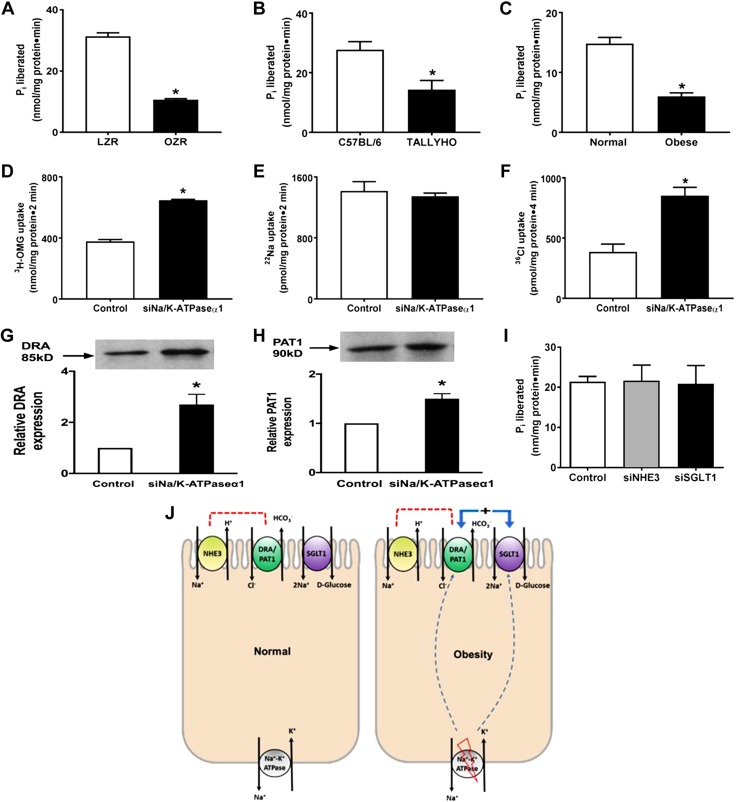
Figure 7.
Obesity-mediated alterations in Na/K-ATPase activity in intestinal epithelial cells. A–C) Na/K-ATPase activity was significantly reduced in villus-cell homogenates obtained from OZRs vs. LZRs (A), TOM vs. C57BL/6 (B), and obese human small intestine vs. normal (C). D–F) Transfection with siRNA for Na/K-ATPase-α1 subunit (siNa/K-ATPase-α1) in rat intestinal epithelial cells significantly stimulated Na-glucose cotransport (D), whereas Na-H exchange remained unaffected (E) as previously demonstrated and reproduced here. Further, Cl-HCO3 exchange was also stimulated (F) in siNa/K-ATPase-α1 IEC-18 cells. G, H) The BBM protein expression of the C-HCO3 exchangers, DRA (G) and PAT1 (H), was significantly increased in siNa/K-ATPase-α1 IEC-18 cells compared with controls. I) Proposed model of deregulation of glucose and Na homeostasis in obesity. J) Normal mammalian intestinal epithelial cells with traditional coupled NaCl absorption via coupling of BBM Na-H and Cl-HCO3 exchange, BBM Na-glucose cotransport and BLM Na/K-ATPase (left panel), and alterations in obesity (right panel) showing inhibition of BLM Na/K-ATPase stimulating BBM Na-glucose cotransport and C-HCO3 exchange, resulting in a novel mechanism of stimulated NaCl absorption, whereas traditional coupled NaCl absorption is maintained, thus resulting in deregulation of glucose and Na homeostasis seen in obesity. All observations are consistent with the observations from all 3 in vivo models of obesity. For all experiments, n represents different studies performed with intestinal cells isolated from different host each time. All experiments in D–H were consistently reproduced at least 3 times, each time with transfected IEC-18 cells revived from a different frozen stock. In G and H, the upper panel is a representative Western blot experiment performed at least 3 times and quantitated in the lower panels; n = 4; pi, P<0.01. *P < 0.01.