Figure 5.
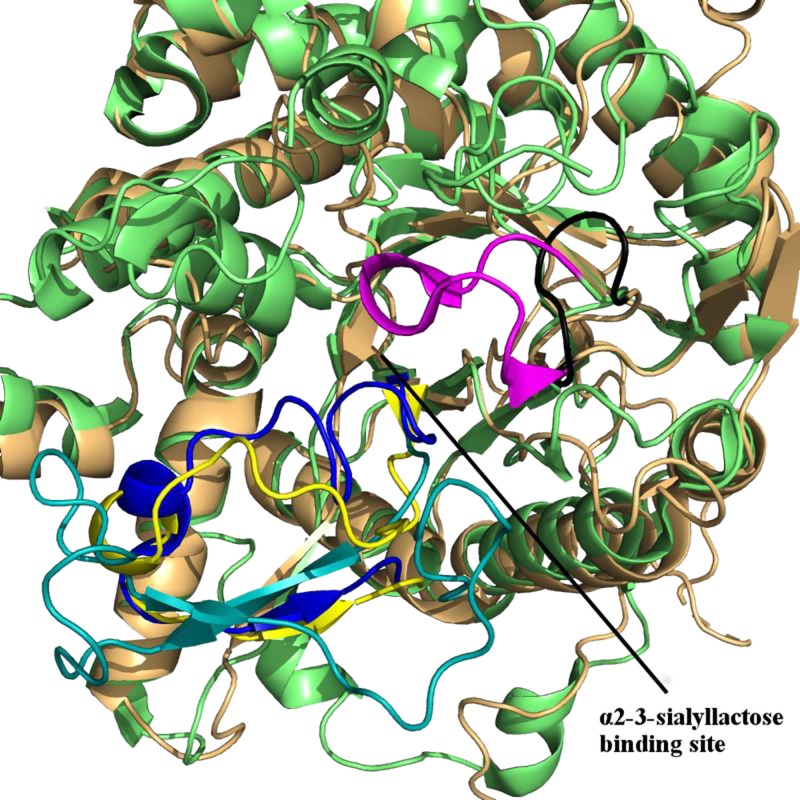
The crystal structure of sKlotho (PDB 5w21) shows that αKL2 (orange) provides a less favorable binding site for α2-3-sialyllactose than αKL1 (green). A 12-residue loop in αKL1 (residues 463–474, magenta) has multiple interactions with the bound α2-3-sialyllactose. The equivalent loop in αKL2 contains only 7 residues (residues 919–925, black) and is oriented away from the proposed binding site. Its orientation renders the central αKL2 core wider and less favorable for binding α2-3-sialyllactose. The αKL1 β6α6 loop (residues 370–393) from the X-ray structure is shown in blue, whereas its location from the MD simulations is in yellow and the equivalent β6α6 loop from αKL2 is in teal. Note that the β6α6 loop of αKL2 is in a nonoccluding conformation.