Table 1.
STs and DSTs in human clinical trials
| Compounds | Chemical Structures | Clinical trial phase | Disease | Sponsor | References |
|---|---|---|---|---|---|
| Mipsagargin (G-202) | 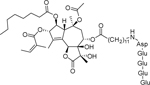 |
Phase 1 | Advanced solid tumors | GenSpera, Inc. | [43] |
| Phase 2 | Chemotherapy-naive metastatic castrate-resistant prostate cancer | GenSpera, Inc. | [44] | ||
| A soluble prodrug of thapsigargin | |||||
| DMAPT (or LC-1) |  |
Phase 1 | Acute myeloid leukemia | Leuchemix Inc. | [45] |
| A water-soluble analog of parthenolide | |||||
| Artesunate | 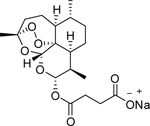 |
Phase 1 | Hepatocellular carcinoma | University Hospital, Ghent | [46] |
| Phase 1 | Solid tumors | Georgetown University | [47] | ||
| Phase 1 | Cervical intraepithelial neoplasia grade 2/3, high-risk HPV (any strain) | Sidney Kimmel Comprehensive Cancer Center |
[48] | ||
| Phase 2 | Colorectal cancer, bowel cancer | St George’s, University of London | [49] | ||
| A semi-synthetic derivative of Artemisinin | Phase 1 | Metastatic breast cancer, locally advanced breast cancer | Heidelberg University | [50] | |
| Artemether | 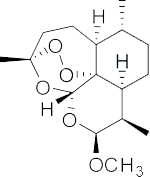 |
Phase 1; Phase 2 | Solid tumors | LondonPharma Ltd. | [51] |
| A semi-synthetic derivative of Artemisinin | |||||
