Figure 1.
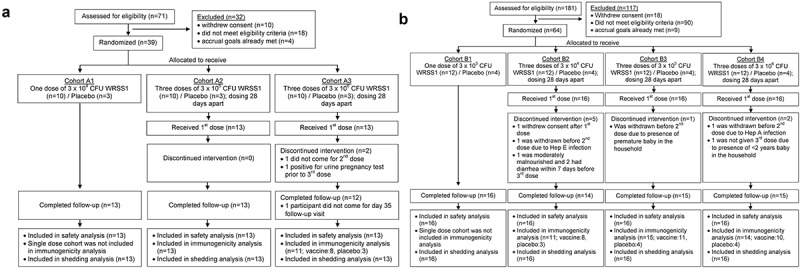
CONsolidated Standards of Reporting Trials (CONSORT) diagram showing screening, enrollment, allocation of vaccine/placebo, completion of intervention, and follow-up status of Bangladeshi (a) Adults and (b) Children participants. Each cohort of 13 adults (10 vaccinees and 3 placebo recipients) received either one dose of 3 × 104 (cohort A1) or three doses of 3 × 105 (cohort A2) or 3 × 106 (cohort A3) CFU of WRSS1 vaccine or placebo. Each cohort of 16 children (12 vaccinees and 4 placebos) received either one dose of 3 × 103 (cohort B1) or three doses of 3 × 104 (cohort B2), 3 × 105 (cohort B3) or 3 × 106 (cohort B4) CFU of vaccine or placebo.
