Figure 2.
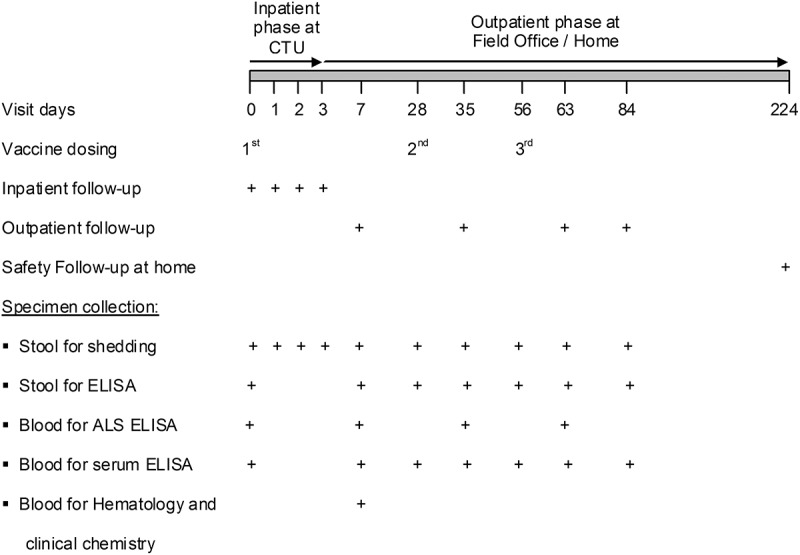
Outline for administration of vaccine/placebo, follow-up and specimen collection. Schedule for multi-dose cohorts (cohorts A2, A3, B2, B3 and B4) is given here. First vaccination and immediate inpatient safety evaluation for 72h was performed at the Clinical Trials Unit (CTU) of icddr,b. The second and third doses were given on an outpatient basis at the field office. Outpatient follow-up at day 7, 35, 63 and 84 were carried out at the field office. Long term safety follow-up took place at day 224 at the participants’ home. cohorts A1 and B1were given single dose on day 0, followed-up on day 7 and 28, and long term safety follow-up was made on day 168.
