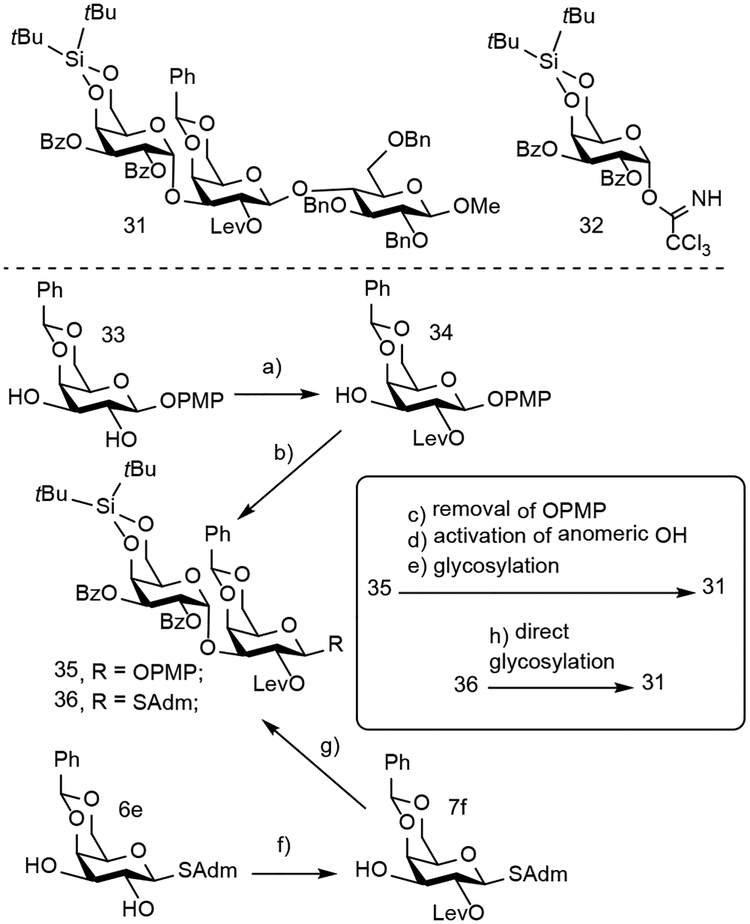
Scheme 3.
Comparison of method efficiency to prepare oligosaccharides using either cation-n directed C2 OH acylation with O-glycosides or site-selective acylation using readily activated S-glycosides.
Reaction conditions: a) (R)-BTM, LevOH, Piv2O, iPr2Et, CHCl3, rt, 85%; b) 32, TMSOTf, CH2Cl2, 0°C-rt, 83%; (c) CAN, CH3CN, H2O, 70%; d) CCl3CN, DBU, 64%; (e) 29e, TMSOTf, CH2Cl2, 0°C-rt, 81%; f) (R)-BTM, LevOH, Piv2O, iPr2Et, CHCl3, rt, 88%; g) 32, TMSOTf, CH2Cl2, 0°C-rt, 62%; (h) 29e, NIS, TMSOTf, CH2Cl2:CH3CN (2:1), −78°C–0°C, 69%