Table 2.
Regiodivergent Arylboration of 1,2-Disubstituted Alkenylarenes
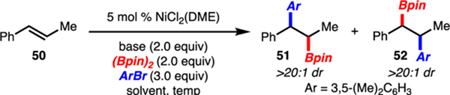 | |||||||
|---|---|---|---|---|---|---|---|
| entry | solvent | temp (°C) | base | yield (%)[a] | 51 | : | 52 |
| 1 | DMA | rt | KOEt | 98 | >99 | : | 1 |
| 2 | toluene:DMA (1:1) | rt | KOEt | 98 | 93 | : | 1 |
| 3 | toluene:DMA (5:1) | rt | KOEt | 42 | 34 | : | 1 |
| 4 | toluene:DMA (10:1) | rt | KOEt | 28 | 14 | : | 1 |
| 5 | toluene:DMA (50:1) | rt | KOEt | 15 | 4.6 | : | 1 |
| 6 | toluene:DMA (160:1) | rt | KOEt | 17 | 2.3 | : | 1 |
| 7 | toluene | rt | KOEt | 15 | 1 | : | 9 |
| 8 | toluene | 60 | KOEt | 55 | 1 | : | 2.2 |
| 9 | toluene | 60 | KOMe | 42 | 1 | : | 5 |
| 10[b] | toluene | 60 | KOMe | 42 | 1 | 36 | |
| 11 [b],[c] | toluene | 60 | KOMe | 45 | 1 | 39 | |
| 12[c],[d] | toluene | 60 | KOMe | 58 | 1 | >99 | |
Reactions performed on 0.2 mmol scale.
Combined yield of 51 and 52 determined by GC using a calibrated internal standard.
ArBr (1.2 equiv).
NiCl2(DME) (15 mol%).
Alkene (2.0 equiv), ArBr, (1.0 equiv).
