Figure 3.
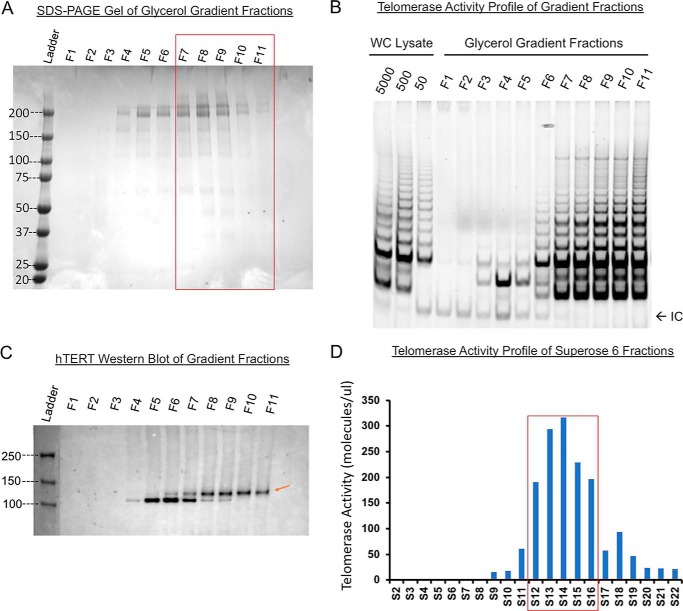
Recombinant telomerase complexes from super-H1299 cells have a major fraction of dimers. Molecular weight marker thyroglobulin (∼669 kDa) was run on a 10–30% glycerol gradient in parallel with recombinant telomerase. A, Coomassie-stained SDS-PAGE assay of thyroglobulin in all 11 gradient fractions (top F1 to bottom F11). Red box marks the fractions where telomerase activity was detected. B, gel-based TRAP assay on gradient fractions. Lysates of super-H1299 fractionated in the glycerol gradient were assayed. Majority of the activity was found in fractions 8–11. C, Western blotting (anti-hTERT) of gradient fractions shows that the recombinant hTERT (red arrow) is primarily in F8–F11. The holoenzyme is thus slightly heavier than 669 kDa. D, recombinant telomerase run through a Superose 6 column. ddTRAP assay of the eluted fractions is plotted against fraction number (1 ml each). Red box marks the major activity containing fractions (S12–S16). Cell equivalency for each fraction was 50 due to the need for better droplet separation in ddPCR. Thyroglobulin run on the same column eluted with its peak in fractions S14–S15, suggesting that the recombinant telomerase holoenzyme complex be heavier than 669 kDa.