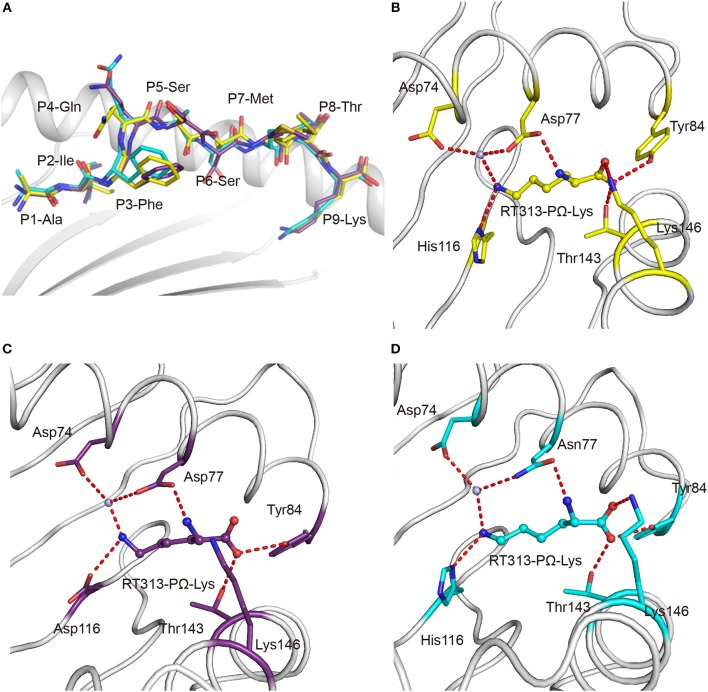
Figure 2.
Similar anchoring modes of A3 supertype peptide in the F pockets of HLA-A*30:01 and HLA-A*30:03. (A) Superposition of RT313 bound to HLA-A*30:01 (yellow), HLA-A*30:03 (cyan), or HLA-A*11:01 (purple). The alignment of these three structures showed a similar overall conformation for RT313 in the peptide-binding grooves. (B–D) The binding modes of the PΩ-Lys residue of RT313 in the F pockets of HLA-A*30:01 (B), HLA-A*11:01 (C), and HLA-A*30:03 (D) are shown. The major residues of the F pockets are shown as sticks while the hydrogen bonds of the F pockets are represented as red dashed lines. PΩ-Lys residues have hydrogen bonds with Asp74 through a water molecule in all three structures. Although HLA-A*30:01 and HLA-A*30:03 contain a His at position 116, which differed from HLA-A*11:01, which had Asp at this position, both His116 and Asp116 could form a hydrogen bond with the positively charged anchor, Lys.