Figure 6.
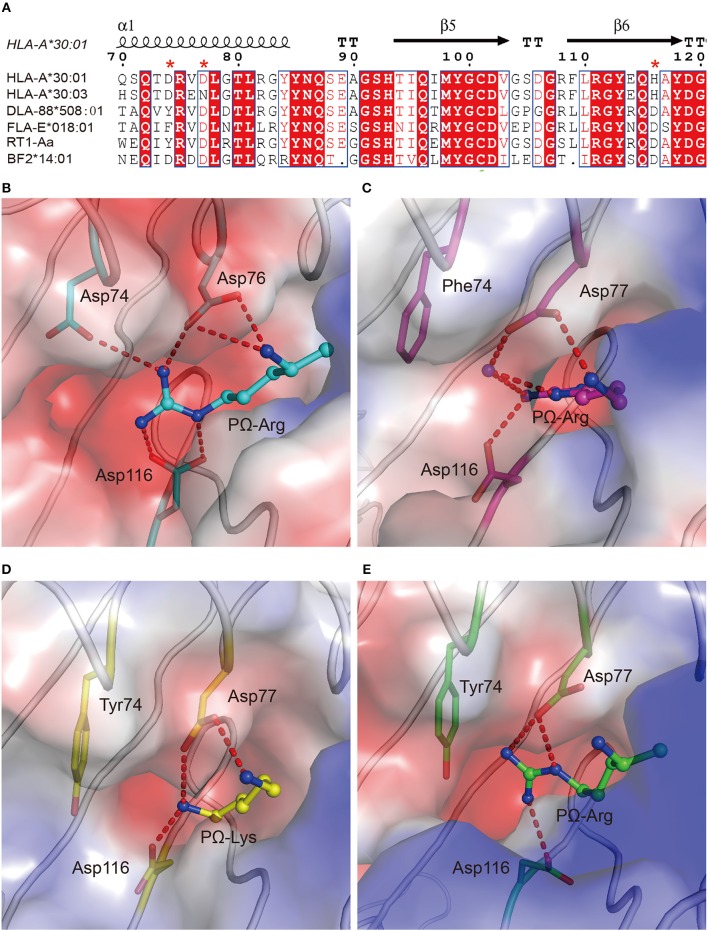
The preference of peptides with PΩ Lys/Arg by MHC I with Asp77 in non-human animals. (A) Structure-based sequence alignment of HLA-A*30:01, HLA-A*30:03, DLA-88*508:01, FLA-E*018:01, RT1-Aa, and BF2*14:01. Cylinders indicate α-helices and black arrows indicate β-strands. Amino acids highlighted in red are completely conserved and those in blue boxes are highly (>80%) conserved. Residue 74, 77, and 116 were labeled with an asterisk. Vacuum electrostatic surface potential of the F pockets of chicken MHC I BF2*14:01 (PDB code: 4CW1) (B), feline MHC I FLA-E*018:01 (5XMF) (C), dog MHC I DLA-88*508:01 (5F1N) (D), and rat MHC I RT1-Aa (1ED3) (E). Residues 74, 77, and 116 are shown as sticks under the vacuum electrostatic surface. The hydrogen bonds between PΩ of peptides and residues 74, 77, or 116 are represented as red dashed lines.