Abstract
Cancer remains a complex disease with increasing global mortality and morbidity. Numerous theories have been established to understand the biological mechanism underlying cancer. One of the most renowned frameworks is the hallmark of cancer proposed by Hanahan and Weinberg that covers ten eminent characteristics of cancer: (i) genome instability and mutation, (ii) sustaining proliferative signaling, (iii) evading growth suppressor, (iv) enabling replicative immortality, (v) resisting cell death, (vi) inducing angiogenesis, (vii) activating invasion and metastasis, (viii) avoiding immune destruction, (ix) tumor-promoting inflammation, and (x) deregulating cellular energetics. These hallmarks provide a rational approach to design an anticancer therapy. In the current review, we summarized specific target molecules on each hallmark of cancer. Further, we evaluated the biological activity of several Indonesia medicinal plants against those specific targets. We explicated the anticancer and chemopreventive activities of some medicinal plants that have been used for centuries by local communities in Indonesia, including Curcuma genus, Brucea javanica, Boesenbergia pandurata, Caesalpinia sappan, and Nigella sativa. Interestingly, these medicinal plants target several hallmarks of cancer, and even Curcuma genus exhibited biological activities that target all hallmarks of cancer. Further, we also discuss several strategies to develop those medicinal plants and/or their active compounds as anticancer and chemopreventive agents.
Keywords: Cancer, Chemoprevention, Indonesia, Medicinal plants
Introduction
Despite current advancement in cancer prevention, treatment, and diagnostic; cancer remains global jeopardy with 70% of cancer-related deaths take place in low- and middle-income countries.1 Characterized by uncontrolled cells proliferation, cancer arises from a genetically transformed cell in a process called carcinogenesis that includes initiation, promotion, and progression stages.2 Moreover, cancer holds diversities for each cancer type with particular molecular mechanisms. A notable work of Hanahan and Weinberg simplified the complexity of cancer into ten properties called ‘the hallmarks of cancer’.3 The long-term carcinogenesis, together with complex molecular characteristics, makes cancer difficult to be cured completely. Therefore, we need a systematic strategy to eradicate cancer based on its specific molecular markers.
The long timeframe between cancer initiation and invasive cancer could be exploited to prevent cancer through cancer chemoprevention, an effort to prevents, inhibits, and/or reverses cancer development.4 For this purpose, various natural products show the biological activity to inhibit cancer progression at certain stages with specific molecular targets (Figure 1). Even more, there are many natural substances performing pleiotropic effects in cancer signaling that may share in some stages of cancer progression, i.e. curcumin and thymoquinone.
Figure 1.
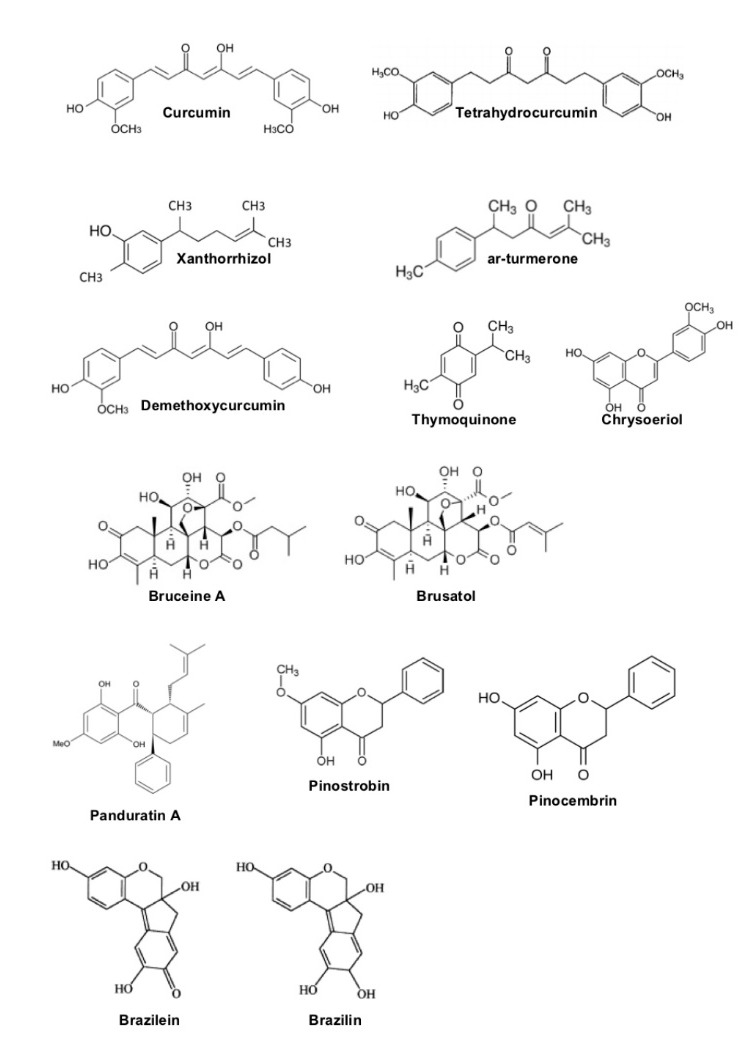
The structure of various natural products exerting chemopreventive effect. The structures of curcumin, tetrahydrocurcumin, xanthorrizol, ar-turmerone, and demethoxycurcumin were adapted from Itokawa et al.5 The structures of bruceine A and brusatol were adapted from Hall et al.6 The structure of thymoquinone was adapted from Bassha et al.7 The structures of panduratin A, pinostrobin, and pinocembrin were adapted from Kiat et al.8 The structures of brazilein and brazilin were adapted from Laksmiani et al.9
Indonesia, a mega-biodiversity country, holds potency to provide chemopreventive agents based on its medicinal plants. Herbal medicine is popular there with around 6000 plant species have been used by Indonesian community for various disease prevention and treatment.10 In this review, we aim to elaborate the potency of medicinal plants widely used in Indonesia as the chemopreventive agents targeted on the hallmarks of cancer. We would focus on some medicinal plants that have been used for centuries in Indonesia, including Curcuma genus, Brucea javanica, Boesenbergia pandurata, Caesalpinia sappan, and Nigella sativa.
Hallmarks of cancer and representation of the Indonesia medicinal plants with specific anti-cancer target
Genome instability and mutation
Cancer begins with unrepaired mutation(s) in the cells, which are accumulated during the time and disrupt normal cell function. In normal cells, various DNA repair pathways rectify almost all DNA mutations, i.e. non-homologues end-joining (NHEJ) and homologous recombination (HR).11 Interestingly, about 25% of human cancers exhibit defects of HR.12 Further exploitation in the defect of DNA repair mechanism offers a potential target for the anticancer drugs, as the prolonged existence of DNA double-strand breaks (DSBs) leads to cellular cytotoxicity.13 Therefore, developing studies assessed the potency of HR inhibitors as anticancer drugs. The target proteins of those HR inhibitors include ataxia-telangiectasia mutated (ATM), checkpoint kinase (CHK) 1 and 2, ataxia telangiectasia and Rad3-related protein (ATR), and Brca1.12,14 Another mechanism of DNA repair is base excision repair, which is facilitated by poly (ADP-ribose) polymerase (PARP).15
Curcumin, from turmeric (Curcuma longa), shows potency to act as a chemopreventive agent targeting genome instability and mutation (Table 1). Curcumin targets 3 major DNA damage repair pathways: non-homologous end joining (NHEJ), homologous recombination repair (HRR), and DNA damage checkpoint response (DDR).16,17 Curcumin suppresses NHEJ through inhibition of histone acetyltransferase activity and suppresses HR pathways by inhibiting Brca1 expression.17 Curcumin also alleviates the activation of ATR-CHK1 signaling.
Table 1. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ genome instability and mutation .
| Molecular target | Plant/compounds | Reference(s) |
| CHK 1 (↓) | Curcumin | 16 |
| ATR (↓) | Curcumin | 17 |
| Histone acetyltransferase (↓) | ||
| Brca1 (↓) |
CHK1: checkpoint kinase 1; ATR: ataxia telangiectasia and Rad3-related protein; Brca1: Breast cancer type 1 susceptibility protein.
↓: indicates down-regulation of a protein and/or pathway.
Sustaining proliferative signaling
Cell proliferation regulators consist of inducers (growth factors, receptor tyrosine kinases/RTKs, transcription factors) and effectors. While the proliferation of normal cells is strictly regulated, cancer cells exhibit aberrant regulation of cell proliferation.18 Cancer cells may overexpress either the receptors or ligands of growth factors, such as androgen receptor (AR) in prostate cancer and estrogen receptor (ER) in breast cancer.19,20 RTKs are also commonly deregulated in the cancer cells, including epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and Bcr-Abl.21,22 In addition, aberrant activation of transcription factors are also important for carcinogenesis. The overactivation of NF-κB plays an extensive role in carcinogenesis.23 The role of c-Myc as a proto-oncogene has been well-established and the overexpression of c-Myc is often associated with poor prognosis in the cancer patients.24 The effector of cell proliferation is cell cycle machinery, which consists of cyclins and cyclin-dependent kinases (CDKs) as the regulatory proteins. About 15%–40% of cancer shows amplification of CCND1, cyclin D1 gene.25 The overexpression of cyclin E associates with trastuzumab resistance in HER2-positive breast cancer patients.26
Various Indonesian medicinal plants show chemopreventive activity by inhibiting the proliferative signaling pathways in cancer cells (Table 2). Thymoquinone selectively inhibits the proliferation of prostate cancer cells by suppressing AR overactivation.27 Curcumin also inhibits AR expression in prostate cancer cells.28 The aqueous extract of B. javanica attenuates EGFR activity in liver cancer and human non-small-lung cancer cells; resulting in the inhibition of cancer proliferation.29,30 Ethanolic extract of C. sappan inhibits HER2 expression in breast cancer cells.31 Interestingly, curcumin shows an impressive activity against various RTKs, including EGFR and HER-2, in colon cancer cells.32
Table 2. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ proliferative signaling .
| Molecular target | Plant/compounds | Reference(s) |
| AR signaling (↓) |
Thymoquinone Curcumin |
27
28 |
| EGFR (↓) | Brucea javanica (aqueous extract) | 29,30 |
| Curcumin | 32 | |
| HER2 (↓) | C. sappan (ethanol extract) | 31 |
| Curcumin | 32 | |
| NF-κB signaling (↓) | Bruceajavanone B | 33 |
| Bruceine A | 33 | |
| Brusatol | 34 | |
| Panduratin A | 35 | |
| c-Myc (↓) | Bruceantin | 36 |
| Cell cycle (CDKs/cyclins) (↓) | Panduratin A (cyclin D1, cyclin E; CDK 2, 4, 6) | 37 |
| Brazilein (cyclin D) | 38 |
AR: androgen receptor; EGFR: epidermal growth factor receptor; HER2: human epidermal growth factor receptor 2; CDK: cyclin-dependent kinase.
↓: indicates down-regulation of a protein and/or pathway.
Bruceajavanone B, bruceine A, and brusatol (from B. javanica) inhibit the activation of NF-κB in leukemia and pancreatic cancer.33,34 Panduratin A also shows its anticancer effect in lung cancer cells by inhibiting the activation of NF-κB.35 Bruceantin down-regulates c-Myc in multiple myeloma cells, leading to the inhibition of cell proliferation and induction of apoptosis.36 Panduratin A down-regulates multiple cell cycle regulatory proteins in prostate cancer cells, including cyclin D1, cyclin E, Cdk 2, Cdk 4, and Cdk 6.37 Brazilein, from C. sappan, down-regulates cyclin D1 and induces cell cycle arrest in G1 phase in MCF-7 breast cancer cells.38
Evading growth suppressor
Tumor suppressors have the eminent roles to inhibit the growth signaling cascades; however, their loss of function is frequently found in the cancer cells. Retinoblastoma protein (pRb) inactivates E2F, which is an important transcription factor for cell cycle progression, DNA replication, DNA damage repair, cell cycle checkpoint, and apoptosis.39 The p53 is another powerful tumor suppressor protein that up-regulates the gene expression of proteins involved in the cell cycle arrest, senescence, and apoptosis; serving as a barrier for the growth of cancer cells.40 Loss or mutation of p53 occurs in various cancers, including in 50% of non-small-cell lung cancer cases and skin cancer, above 70% of small-cell lung cancers, and almost 100% in high-grade serous carcinoma of the ovary.41-43
Phosphatase and tensin homolog (PTEN) is a phosphatase that serves as an important inhibitor for PI3K/Akt/mTOR pathway. Loss of PTEN occurs in various sporadic tumors.44,45 Other prominent growth suppressors are the Cdk inhibitors (Cdki), such as p16, p21, and p27. These proteins are activated by p53 and inhibit cell cycle progression from G1 to S phase.46
Various natural compounds exhibit the ability to restore the level and/or function of tumor growth suppressors (Table 3). Aqueous extract of B. javanica increases the level of p53 in breast cancer and cervical cancer cells.47 Thymoquinone increases the level of p53 in cervical cancer cells and up-regulates PTEN in both mRNA and protein level in doxorubicin resistance-breast cancer cells.48,49 Curcumin up-regulates the expression of multiple tumor growth suppressors, including p16/INK4a, p21/WAF1/CIP1, and p27/KIP1; as well as inhibits hyperphosphorylation of retinoblastoma (Rb) protein.50
Table 3. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ growth suppressors .
| Molecular target | Plant/compounds | Reference(s) |
| p53 (↑) | Brucea javanica (aqueous extract) | 47 |
| Thymoquinone | 48 | |
| PTEN (↑) | Thymoquinone | 49 |
| pRb(↑) | Curcumin | 50 |
| Cdki (↑) | Curcumin | 50 |
PTEN: phosphatase and tensin homolog; pRb: retinoblastoma protein; Cdki: cyclin-dependent kinase inhibitor.
↑: indicates up-regulation of a protein and/or pathway.
Enabling replicative immortality
By shortening of DNA telomere, normal cells would enter a phase called replicative senescence where they could not further proliferate. However, cancer cells escape from this event through various mechanisms; thus are able to proliferate continuously. Telomerase, a complex of enzymes repressed in normal cells, is activated in the cancer cells and preserves the telomere of cancer cells.51 Telomerase complex consists of various enzymes; one of the most important is telomerase reverse transcriptase (TERT; hTERT for human) that becomes the rate-limiting step in telomerase activity.52 Telomerase is found in about 85% to 90% of all malignant tumors and become an interesting target for the anticancer drugs.53 Several natural compounds show anticancer activity by targeting replicative immortality of cancer cells (Table 4). Methanolic extract of C. sappan was shown to inhibit telomerase activity in oral carcinoma and osteosarcoma cells.54 Curcumin also inhibits telomerase activity, which mediated by proteasome induced-degradation of hTERT.55
Table 4. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ replicative immortality .
| Molecular target | Plant/compounds | Reference(s) |
| Telomerase (↓) | C. sappan (methanol extract) | 54 |
| Curcumin | 55 | |
| hTERT (↓) | Curcumin | 55 |
hTERT: human telomerase reverse transcriptase. ↓: indicates down-regulation of a protein and/or pathway.
Resisting cell death
Beside proliferate uncontrollably; cancer cells are also able to resist cell death (apoptosis). Apoptosis is initiated through two main pathways: intrinsic and extrinsic pathways. The intrinsic pathway is closely related with mitochondria permeabilization and regulated by the homeostasis of pro- and anti-apoptotic proteins, i.e. Bak, Bax, Bim (pro-apoptotic) or Bcl-2, Bcl-xL, Mcl-1 (anti-apoptotic).56 The extrinsic pathway starts with the activation of death receptors in the cell membrane, i.e. TNF-related apoptosis-inducing ligand (TRAIL) and Fas receptor.57 Those death receptors are activated by several ligands: TNF for TRAIL and Fas ligand (FasL) for Fas. The agonist of death receptor is promising to be developed as the anticancer agents.58
Various natural compounds are reported to overcome apoptosis resistance in cancer cells (Table 5). Brucein D, brusatol, brazilin, pinostrobin, and curcumin were reported to down-regulate Bcl-2.34,59-62 Curcumin suppresses important anti-apoptotic protein Mcl-1.60 Panduratin A up-regulates both Fas and TRAIL in prostate cancer cells.37
Table 5. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ cell death .
| Molecular target | Plant/compounds | Reference(s) |
| Bcl-2 (↓) | Brusatol | 34 |
| Brucein D | 59 | |
| Curcumin | 60 | |
| Brazilin | 61 | |
| Pinostrobin | 62 | |
| Mcl-1 (↓) | Curcumin | 60 |
| Fas (↑) | Panduratin A | 37 |
| TRAIL (↑) | Panduratin A | 37 |
Bcl-2: B-cell lymphoma 2; Mcl-1: Induced myeloid leukemia cell differentiation protein; TRAIL: TNF-related apoptosis-inducing ligand.
↓: indicates down-regulation of a protein and/or pathway.
↑: indicates up-regulation of a protein and/or pathway.
Inducing angiogenesis
Cancer cells develop blood vessels independently from normal cellular physiology to support their need for nutrition and oxygen in a process called angiogenesis. During angiogenesis, cancer cells secrete various pro-angiogenic factors that stimulate endothelial cells to grow and produce various digestive enzymes.63 These factors include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and fibroblast growth factor 2 (FGF-2).
Without enough supply of oxygen from blood vessels, cancer cells suffer from a hypoxia condition. In this situation, hypoxia-inducible factor-1α (HIF-1α) is activated. And induces the expression of various genes that promote the mitogenic and migratory activities of endothelial cells.64 Hence, HIF-1α is an interesting molecule to target cancer angiogenesis.
Several natural compounds are reported to interfere angiogenesis in cancer cells (Table 6). Studies found that thymoquinone suppresses the level of VEGFR2; while ar-turmerone down-regulates VEGFR3.65,66 Curcumin down-regulates the transcriptional activity of HIF-1α under hypoxia, resulting in the suppression of VEGF level.67 Tetrahydrocurcumin decreases VEGF level in osteosarcoma cells and down-regulates HIF-1α, resulting in mesenchymal-epithelial transition/MET.68
Table 6. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ angiogenesis .
| Molecular target | Plant/compounds | Reference(s) |
| VEGFR (↓) | ar-turmerone | 65 |
| thymoquinone | 66 | |
| VEGF (↓) | Curcumin | 67 |
| HIF-1α (↓) | Curcumin | 67 |
| Tetrahydrocurcumin | 68 |
VEGFR: vascular endothelial growth factor receptor; VEGF: vascular endothelial growth factor; HIF-1α: hypoxia-inducible factor-1α.
↓: indicates down-regulation of a protein and/or pathway
Activating invasion and metastasis
Metastasis is responsible for >90% mortality of patients with solid tumors.69 In order to achieve metastasis, cancer cells produce various proteins that play important roles in cell-cell adhesion, cell-matrix adhesion, cellular migration, and epithelial-mesenchymal transition (EMT). E-cadherin is a key protein regulating cell-cell adhesion through the formation of the cell-cell junction. Loss of E-cadherin is found in the progression of tumor malignancy of most epithelial tumors.70 Down-regulation of E-cadherin occurs via various mechanisms: genetic or epigenetic mechanism, transcriptional suppression, proteolytic degradation, and modulation of several RTKs.71 Beta-catenin, while being an important protein for cell-cell adhesion, also serves as an oncogenic protein. Nuclear localization of β-catenin induces EMT resulting in metastasis.72 In cell-matrix adhesion, integrin plays a significant role to facilitate the interaction between cells and extracellular matrix (ECM).73 Other key proteins for invasion and metastasis are matrix metalloproteinases (MMPs). MMP-2 and MMP-9 degrade ECM components to facilitate cancer cells invasion and migration.74 Moreover, the overexpression of several MMPs could induce EMT.75
Numerous natural compounds exhibit inhibitory effect on invasion and metastasis (Table 7). B. javanica oil inhibits metastasis by up-regulating integrin.76 Thymoquinone reduces the expression of MMP-9, hence suppresses metastasis to multiple vital organs, including lungs, brain, and bone in the animal model of cancer.66 Thymoquinone down-regulates the expression of HER-2 and reduces the motility and migration of a highly metastatic pancreatic cancer cell line.77
Table 7. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ invasion and metastasis .
| Molecular target | Plant/compounds | Reference(s) |
| E-cadherin (↑) | Tetrahydrocurcumin | 68 |
| Curcumin | 85 | |
| MMP-2 (↓) | C. sappan (ethyl acetate fraction) | 78 |
| Brazilein | 79 | |
| Panduratin A | 35,82 | |
| ar-turmerone | 65 | |
| Tetrahydrocurcumin | 79 | |
| MMP-9 (↓) | C. sappan (ethyl acetate fraction) | 78 |
| Brazilein | 80 | |
| Brazilin | 81 | |
| thymoquinone | 66 | |
| xanthorrhizol | 83 | |
| ar-turmerone | 65,86 | |
| Tetrahydrocurcumin | 68 | |
| Demethoxycurcumin | 84 | |
| HER2 (↓) | C. sappan (ethyl acetate fraction) | 78 |
| Thymoquinone | 77 | |
| β-catenin (↓) | Tetrahydrocurcumin | 68 |
| Integrin (↓) | Brucea javanica (oil) | 76 |
MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; HER2: human epidermal growth factor receptor 2.
↓: indicates down-regulation of a protein and/or pathway; ↑: indicates up-regulation of a protein and/or pathway.
Ethyl acetate fraction of C. sappan decreases the protein level of MMP-2, MMP-9, and HER-2; thus inhibits cell migration in HER-2 overexpressed-breast cancer cells.78 Brazilein down-regulates MMP-2 and MMP-9.79,80 Whereas, brazilin decreases 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced invasion in MCF-7 breast cancer cells through down-regulation of MMP-9 expression.81
Panduratin A suppresses the secretion and activation of MMP-2, resulting in the inhibition of endothelial cell migration, invasion, and morphogenesis in HUVEC cells and zebrafish embryo.82 In addition, the sub-toxic dose of panduratin A is sufficient to down-regulate MMP-2 in lung cancer cells.35 Xanthorrizol and demethoxycurcumin inhibits metastasis in mouse lung metastasis model and MDA-MB-231 breast cancer cells, respectively, through down-regulation of MMP-9.83,84 Curcumin increases E-cadherin.85 Tetrahydrocurcumin inhibits the migration and invasion of osteosarcoma cell line by increasing E-cadherin and suppressing MMP-2, MMP-9, and β-catenin.68 Finally, the sub-toxic dose of ar-turmerone inhibits metastasis through down-regulation of MMP-2 and MMP-9.65,86
Avoiding immune destruction
Cancer cells can avoid immune system through several ways, including (i) modification of immune regulatory cells, (ii) defective antigen presentation in the tumor cells, (iii) immune suppressive mediators, (iv) tolerance and immune deviation, and (v) induce apoptosis in immune cells.87 T regulatory (Treg) cell is a suppressor of the immune system. Tumor cells increase the suppressive activity of Treg and monoclonal antibody against Treg decreases tumor development.88,89 Natural killer (NK) cells also can be exploited to target cancer cells. NK cells were shown to control and/or eradicate some of the human hematopoietic tumors, as well as able to eliminate metastasizing cells and small tumor grafts.90,91 T cells, both helper and cytotoxic cells, were shown to have a positive impact on eliminating cancer. High expression of the Th1 cluster and CD8+ are positively correlated with prolonged disease-free survival in patients with colon cancer.92 At the molecular level, IL-12 shows various immunomodulatory activity, such as induces interferon-γ (IFN-γ) secretion and promotes the maturation of cytotoxic T cells.93
Nigella sativa and Curcuma genus show immunomodulatory activities against cancer cells (Table 8). The aqueous extract of N. sativa enhances the cytotoxicity of natural killer (NK) cells against YAC-1 tumor cells, indicating its potency as the stimulant for NK cells antitumor activity.94 Low concentration of thymoquinone increases the activation of CD8+ T cells and might beneficial for conditioning T cells in vitro, which will be used in T-cell therapy against cancer.95
Table 8. Indonesian plants and/or their components that show chemopreventive activity targeting cancers’ resistance to immune system .
| Molecular target | Plant/compounds | Reference(s) |
| NK cell activity(↑) | N. sativa (aqueous extract) | 94 |
| Th1 response (↑) | Curcumin | 96 |
| Treg lymphocytes (↓) | Curcumin | 96 |
| T-cell killer activity (↑) | Thymoquinone | 95 |
| Curcumin | 96 | |
| IL-12 (↑) | ar-turmerone | 97 |
NK: natural killer; Th1: T helper cell type 1; Treg: regulatory, IL-12: interleukin 12.
↓: indicates down-regulation of a protein and/or pathway; ↑: indicates up-regulation of a protein and/or pathway.
Curcumin shows extensive activity against various types of T cells.96 Curcumin also enhances the response of Th1 (T helper cells) and the cytotoxicity of T killer cells. In addition, curcumin down-regulates Treg, a suppressor of the immune system. Ar-turmerone, another compound isolated from Curcuma genus, has been shown to increase the level of IL-12 in dendritic cells, which could be beneficial for the anticancer immunotherapy.97
Tumor-promoting inflammation
About 25% of tumor is closely related to chronic inflammation as chronic inflammation promotes tumor cell survival, proliferation, invasion, angiogenesis, metastasis, chemoresistance, and radioresistance.98,99 Moreover, chronic inflammation also may generate reactive oxygen species (ROS) and reactive nitrogen species that could induce the initiation and/or promotion of carcinogenesis.100 Therefore, tumor inflammation is a desirable target for a chemopreventive agent.
NF-κB serves as a transcription factor for various pro-inflammatory enzymes and cytokines.101 However, as NF-κB also closely related to the activation of immune system, NF-κB inhibitors should be designed carefully so that it does not impair the immune system.102
Cyclooxygenase-2 (COX-2) is a well-known enzyme responsible for inflammation events as it mediates the synthesis of pro-inflammatory molecule PGE2. Overexpression of COX-2 was found in various cancer tissues and selective inhibition of COX-2 might be beneficial for the prevention of cancer.103-105 Another important enzyme for inflammation is nitric oxide synthase (iNOS) that catalyzes the production of nitric oxide (NO), a potent pro-inflammatory mediator.106
Cytokines, such as tumor necrosis α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6), are also shown to correlate with tumor-promoting inflammation. TNF-α supports cancer initiation since it stimulates the production of genotoxic molecules, such as ROS and NO. TNF-α and IL-1β also develop a positive feedback loop with NF-κB, resulting in the sustained chronic inflammation in the tumor cells.107 IL-6, another type of cytokine, promotes apoptosis resistance in tumor cells during the inflammatory process.108
Various natural compounds exhibit anti-inflammatory activity that might beneficial to counteract tumor-promoting inflammation (Table 9). Panduratin A suppresses COX-2 expression level in colon cancer cells.109 Brazilin suppresses lipopolysaccharide-stimulated iNOS in RAW 264.7 macrophage cells; as well as inhibits the DNA binding activity of NF-κB.110 B. javanica oil has been shown to down-regulate the expression of COX-2 and p65, an active subunit of NF-κB.111
Table 9. Indonesian plants and/or their components that show chemopreventive activity targeting tumor-promoting inflammation .
| Molecular target | Plant/compounds | Reference(s) |
| COX-2 (↓) | Brucea javanica oil | 111 |
| Panduratin A | 109 | |
| Thymoquinone | 66,112 | |
| Xanthorrhizol | 113 | |
| Curcumin | 32 | |
| iNOS (↓) | Xanthorrhizol | 113 |
| NF-κB signaling (↓) | Brucea javanica oil | 111 |
| Brazilin | 110 | |
| Thymoquinone | 112 | |
| Curcumin | 114 | |
| TNF-α (↓) | N. sativa (aqueous extract) | 94 |
| Curcumin | 114 | |
| IL-6 (↓) | N. sativa (aqueous extract) | 94 |
| Curcumin | 114 | |
| IL-1b (↓) | Thymoquinone | 112 |
COX-2: cyclooxygenase 2; iNOS: nitric oxide synthase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-α: tumor necrosis factor α; IL-6: interleukin 6; IL-1b: interleukin 1b.
↓: indicates down-regulation of a protein and/or pathway.
The aqueous extract of N. sativa suppresses the secretion of IL-6 and TNF-α in primary macrophages cells.94 Thymoquinone decreases the expression level of COX-2 in pancreatic cancer and breast cancer cells.66,112 Furthermore, thymoquinone decreases the level of IL-1β, as well as inhibits the activation of NF-κB.112
Xanthorrizol inhibits the enzyme activity of COX-2 and iNOS in macrophage cells.113 Curcumin has been reported to suppress various inflammatory cytokines, including TNF-α, IL-1, IL-2, IL-5, IL-6, IL-8, IL-12, and IL-18. Curcumin is also a potent inhibitor of NF-κB transcription factor114 and decreases the expression level of COX-2.32
Deregulating cellular energetics
While glycolysis usually occurs in the normal cells under anaerobic condition, cancer cells are able to reprogram their energy metabolism mainly into aerobic glycolysis; the phenomenon that is known as the ‘Warburg effect’.115 Hypoxia is suspected as the basis of tumor cells metabolic reprogramming. When hypoxia occurs, the transcription factor HIF-1α is activated and induces the expression genes involving in glycolysis, including glucose transporter 1 (GLUT1), glucose transporter 3, hexokinase 2, pyruvate kinase 2, lactate dehydrogenase 5, and pyruvate dehydrogenase kinase 1 (PDK1).116 Therefore, HIF-1α serves as a key molecule for the anticancer drugs targeting cancer metabolism.
Cancer cells also suffer from a high level of ROS that is closely related to metabolism dysregulation in cancer cells. While a moderate level of ROS activates the expression of pro-survival genes, such as HIF1A andGLUT1, a high level of ROS in cancer cells trigger metabolism dysregulation and protein translation, resulting in the increase of ROS production.117 As excessive ROS level is toxic to cells, cancer cells upregulate various antioxidant systems, such as glutathione and thioredoxin antioxidant pathways.118 Developing studies reported that modulating ROS level is a potential strategy to eliminate various cancer cells. The combination of glycolysis inhibitor or glucose deprivation with inhibition of antioxidant systems, including glutathione and thioredoxin, exhibits synergistic anticancer effect breast and prostate cancer cells.119
Various compounds also exhibit anticancer activity targeting cancer cell energetic machinery (Table 10). A recent study showed that brusatol induces HIF-1α degradation in colon cancer.120 Furthermore, brusatol pre-treatment under hypoxia condition down-regulates the expression of HIF-1α downstream genes, including GLUT1 and PDK1. Curcumin blocks glucose uptake in GLUT1 expressing cells up to 86%.121
Table 10 . Indonesian plants and/or their components that show chemopreventive activity targeting deregulating cellular energetics induced by cancer cells .
| Molecular target | Plant/compounds | Reference(s) |
| HIF-1α (↓) | Brusatol | 120 |
| GLUT1(↓) | Curcumin | 121 |
| Brusatol | 120 | |
| PDK1 (↓) | Brusatol | 120 |
| ROS (↑) | Brucein D | 122, 123 |
| Xanthorrhizol | 124 | |
| Curcumin | 125 |
HIF-1α: hypoxia-inducible factor-1α; GLUT1: Glucose transporter 1; PDK1: pyruvate dehydrogenase kinase 1; ROS: reactive oxygen species.
↓: indicates down-regulation of a protein and/or pathway;
↑: indicates up-regulation of a protein and/or pathway.
Brucein D generates superoxide and exhibits cytotoxicity in pancreatic cancer cells, but not in non-tumorigenic cells.122,123 Xanthorrizol and curcumin also exhibits pro-oxidative activity. Xanthorrhizol elevates ROS level in oral squamous cell carcinoma cells and concurrent treatment with antioxidant partly reverses the cytotoxic activity of xanthorrhizol.124 Recent study showed that curcumin directly binds to various enzymes involving in ROS metabolic pathway; hence elevates ROS level in cancer cells and ultimately eliminates cancer cells.125
Discussion
Indonesian plants and their bioactive components show promising activity as the anticancer and chemopreventive agents targeting the complex system of cancer. After a thorough investigation, we revealed the activity of Indonesian plants and their components against ten hallmarks of cancer (Tables 1-10). We could see that some constituents target more than one hallmark of cancer, even interfere with all hallmarks of cancer. Such ‘powerful’ compounds are curcumin (10 hallmarks), thymoquinone (7 hallmarks), and panduratin A (5 hallmarks). Despite their extensive targets on cancer cells, curcumin, thymoquinone, and panduratin A remain selective to eliminate cancer cells compared to the non-cancerous cells.32 These studies strengthen the urgency to develop natural compounds from Indonesian plants for clinical use in cancer therapy. However, curcumin and thymoquinone exert poor water solubility (<1.0 mg/mL), while the water solubility of panduratin A has not been well-established yet126,127; leading to a problem for drug formulation. Hence, scientists are concerting their effort to solve this problem, i.e. use curcumin and thymoquinone as the lead compounds to design the more water-soluble drugs or formulate these compounds with various techniques to increase their water solubility.
In their renowned paper about hallmarks of cancer, Hanahan and Weinberg acknowledge that combination chemotherapy (co-chemotherapy) is the key to effective cancer treatment. However, the co-chemotherapy strategy should be formulated on rational cancer targets by targeting multiple pathways or hallmarks of cancer.3 Targeting only one pathway or feature of cancer risks in cancer resistance and therefore, integrative and broad-spectrum co-chemotherapy serves as a potential strategy to combat cancer.128 Even though some of the plants or compounds do not act as a broad targeting-agent like curcumin, they are still promising to be developed as the anticancer and chemopreventive agents. B. javanica and its constituent exhibit their anticancer activities mostly via targeting cancer cells proliferative signaling (Table 2); whereas C. sappan and its constituents are potent anti-metastatic agents (Table 7). Meanwhile, Curcuma genus and N. sativa show promising activity against tumor inflammation (Table 9). Hence, further study could be conducted to determine their best combination as an integrative and broad-spectrum co-chemotherapy. To achieve that goal, the researchers should evaluate the synergistic anticancer effect of the combination of extract, fraction, or even pure compounds of various plants in an integrative experimental model of cancer.
Conclusion
In summary, Curcuma genus, B. javanica, B. pandurata, C. sappan, and N. sativa show extensive anticancer and chemopreventive activities against various hallmarks of cancer. We recognize that only limited plants and compounds could be illustrated in this current article due to space limitation. Therefore, further experimental research and systematic reviews are quintessential to elucidate the anticancer properties of Indonesian plants, as well as develop them to be used clinically for cancer patients.
Ethical Issues
Not applicable
Conflict of Interest
There is no conflict of interest in this study
References
- 1.Making cancer data count. Lancet. 2014;383(9933):1946. doi: 10.1016/s0140-6736(14)60939-9. [DOI] [PubMed] [Google Scholar]
- 2.Pitot HC. The molecular biology of carcinogenesis. Cancer. 1993;72(3 Suppl):962–70. doi: 10.1002/1097-0142(19930801)72:3+<962::aid-cncr2820721303>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109(1):1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itokawa H, Shi Q, Akiyama T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chin Med. 2008;3:11. doi: 10.1186/1749-8546-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall IH, Lee KH, Imakura Y, Okano M, Johnson A. Anti-inflammatory agents III: Structure-activity relationships of brusatol and related quassinoids. J Pharm Sci. 1983;72(11):1282–4. doi: 10.1002/jps.2600721111. [DOI] [PubMed] [Google Scholar]
- 7.Basha LIA, Rashed MS, Aboul-Enein HY. TLC assay of thymoquinone in black seed oil (Nigella sativa Linn) and identification of dithymoquinone and thymol. J Liq Chromatogr. 1995;18(1):105–15. doi: 10.1080/10826079508009224. [DOI] [Google Scholar]
- 8.Kiat TS, Pippen R, Yusof R, Ibrahim H, Khalid N, Rahman NA. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L), towards dengue-2 virus NS3 protease. Bioorg Med Chem Lett. 2006;16(12):3337–40. doi: 10.1016/j.bmcl.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Laksmiani NPL, Susidarti RA, Meiyanto E. Brazilein Increased Cytotoxic Activity of Doxorubicin on MCF-7/DOX Cells. Indonesian Journal of Cancer Chemoprevention. 2015;6(2):58–63. [Google Scholar]
- 10.Yaman E, Elfahmi Elfahmi, Woerdenbag HJ, Kayser O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J Herb Med. 2014;4(2):51–73. doi: 10.1016/j.hermed.2014.01.002. [DOI] [Google Scholar]
- 11.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 12.Pfäffle HN ZL, Willers H. Targeting homologous recombination repair in cancer In: Kelley MR, Fishel ML, eds DNA repair and cancer therapy: molecular targets and clinical applications. Academic Press. 2016:119–60. [Google Scholar]
- 13.Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol. 2003;66(8):1547–54. doi: 10.1016/S0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson LR, Chen H, Collins AR, Connell M, Damia G, Dasgupta S. et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35 Suppl:S5–s24. doi: 10.1016/j.semcancer.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogiwara H, Yokota J, Kohno T. Abstract B65: Curcumin, a novel inhibitor of ATR-CHK1 pathway, suppresses homologous recombination and DNA damage checkpoint and enhances the sensitivity to PARP inhibitors. Mol Cancer Ther. 2011;10(11 Suppl):B65–B. doi: 10.1158/1535-7163.targ-11-b65. [DOI] [Google Scholar]
- 17.Ogiwara H, Ui A, Shiotani B, Zou L, Yasui A, Kohno T. Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis. 2013;34(11):2486–97. doi: 10.1093/carcin/bgt240. [DOI] [PubMed] [Google Scholar]
- 18. Griffiths AJF, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM, et al. Cancer: the genetics of aberrant cell control. An introduction to genetic analysis. 7th ed. New York: W. H. Freeman; 2000.
- 19.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R. et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 20.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97(3 Suppl):825–33. doi: 10.1002/cncr.11126. [DOI] [PubMed] [Google Scholar]
- 21.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. doi: 10.1016/S0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 22.Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J. et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35 Suppl:S25–s54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao G, Fu J. NF-kappaB and cancer: a paradigm of Yin-Yang. Am J Cancer Res. 2011;1(2):192–221. [PMC free article] [PubMed] [Google Scholar]
- 24.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2(10):764–76. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 25.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–72. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 26.Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J. et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011;108(9):3761–6. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaseb AO, Chinnakannu K, Chen D, Sivanandam A, Tejwani S, Menon M. et al. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67(16):7782–8. doi: 10.1158/0008-5472.can-07-1483. [DOI] [PubMed] [Google Scholar]
- 28.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13(4):343–9. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- 29.Chen JH, Kim SH, Fan PW, Liu CY, Hsieh CH, Fang K. The aqueous extract of Chinese medicinal herb Brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Des Devel Ther. 2016;10:2003–13. doi: 10.2147/dddt.s107909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Liu CY, Fan PW, Hsieh CH, Lin HY, Lee MC. et al. The aqueous extract of Brucea javanica suppresses cell growth and alleviates tumorigenesis of human lung cancer cells by targeting mutated epidermal growth factor receptor. Drug Des Devel Ther. 2016;10:3599–609. doi: 10.2147/dddt.s117443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rachmady R, Muntafiah L, Rosyadi F, Sholihah I, Handayani S, Jenie RI. Antiproliferative effect of secang heartwood ethanolic extract (Caesalpinia sappan L) on HER2-positive breast cancer cells. Indonesian Journal of Cancer Chemoprevention. 2016;7(1):1–5. doi: 10.14499/indonesianjcanchemoprev7iss1pp1-5. [DOI] [Google Scholar]
- 32.Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010;30(2):319–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JA, Lau EK, Pan L, De Blanco EJ. NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species. Anticancer Res. 2010;30(9):3295–300. [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Y, Ye W, Huang C, Lou B, Zhang J, Yu D. et al. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-kappab/Stat3/Bcl-2 signaling pathway. Biochem Biophys Res Commun. 2017;487(4):820–6. doi: 10.1016/j.bbrc.2017.04.133. [DOI] [PubMed] [Google Scholar]
- 35.Cheah SC, Lai SL, Lee ST, Hadi AH, Mustafa MR. Panduratin A, a possible inhibitor in metastasized A549 cells through inhibition of NF-kappa B translocation and chemoinvasion. Molecules. 2013;18(8):8764–78. doi: 10.3390/molecules18088764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuendet M, Christov K, Lantvit DD, Deng Y, Hedayat S, Helson L. et al. Multiple myeloma regression mediated by bruceantin. Clin Cancer Res. 2004;10(3):1170–9. doi: 10.1158/1078-0432.CCR-0362-3. [DOI] [PubMed] [Google Scholar]
- 37.Yun JM, Kweon MH, Kwon H, Hwang JK, Mukhtar H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis. 2006;27(7):1454–64. doi: 10.1093/carcin/bgi348. [DOI] [PubMed] [Google Scholar]
- 38.Tao LY, Li JY, Zhang JY. Brazilein, a compound isolated from Caesalpinia sappan Linn, induced growth inhibition in breast cancer cells via involvement of GSK-3beta/beta-Catenin/cyclin D1 pathway. Chem Biol Interact. 2013;206(1):1–5. doi: 10.1016/j.cbi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29(8):409–17. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harb Perspect Biol. 2010;2(8):a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat. 2003;21(3):229–39. doi: 10.1002/humu.10177. [DOI] [PubMed] [Google Scholar]
- 42.Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Hum Mutat. 2003;21(3):217–28. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221(1):49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern HM, Gardner H, Burzykowski T, Elatre W, O’Brien C, Lackner MR. et al. PTEN loss is associated with worse outcome in HER2-amplified breast cancer patients but is not associated with trastuzumab resistance. Clin Cancer Res. 2015;21(9):2065–74. doi: 10.1158/1078-0432.ccr-14-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nita ME, Alves VA, Carrilho FJ, Ono-Nita SK, Mello ES, Gama-Rodrigues JJ. Molecular aspects of hepatic carcinogenesis. Rev Inst Med Trop Sao Paulo. 2002;44(1):39–48. doi: 10.1590/S0036-46652002000100007. [DOI] [PubMed] [Google Scholar]
- 47.Gao H, Lamusta J, Zhang WF, Salmonsen R, Liu Y, O’Connell E. et al. Tumor cell selective cytotoxicity and apoptosis induction by an herbal preparation from Brucea javanica. N Am J Med Sci (Boston) 2011;4(2):62–6. doi: 10.7156/v4i2p062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng WK, Yazan LS, Ismail M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol In Vitro. 2011;25(7):1392–8. doi: 10.1016/j.tiv.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 49.Arafa el SA, Zhu Q, Shah ZI, Wani G, Barakat BM, Racoma I. et al. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat Res. 2011;706(1-2):28–35. doi: 10.1016/j.mrfmmm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6(23):2953–61. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 51.Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33(2):123–9. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- 52.Poole JC, Andrews LG, Tollefsbol TO. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT) Gene. 2001;269(1-2):1–12. doi: 10.1016/S0378-1119(01)00440-1. [DOI] [PubMed] [Google Scholar]
- 53.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6(6):584–93. doi: 10.1158/2159-8290.cd-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JS, Kim YG, Kim JH. Studies on anticancer effects of extracts Caesalpinia sappan on oral carcinoma and osteosarcoma cells. J Korean Assoc Oral Maxillofac Surg. 2001;27(4):281–8. [Google Scholar]
- 55.Lee JH, Chung IK. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 2010;290(1):76–86. doi: 10.1016/j.canlet.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 56.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 57.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13(2):135–47. doi: 10.1016/S1044-579X(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 58.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16(6):1701–8. doi: 10.1158/1078-0432.ccr-09-1692. [DOI] [PubMed] [Google Scholar]
- 59.Lau ST, Lin ZX, Liao Y, Zhao M, Cheng CH, Leung PS. Bruceine D induces apoptosis in pancreatic adenocarcinoma cell line PANC-1 through the activation of p38-mitogen activated protein kinase. Cancer Lett. 2009;281(1):42–52. doi: 10.1016/j.canlet.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Cheng CY, Lin YH, Su CC. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int J Mol Med. 2010;26(5):673–8. doi: 10.3892/ijmm_00000513. [DOI] [PubMed] [Google Scholar]
- 61.Kim B, Kim SH, Jeong SJ, Sohn EJ, Jung JH, Lee MH. et al. Brazilin induces apoptosis and G2/M arrest via inactivation of histone deacetylase in multiple myeloma U266 cells. J Agric Food Chem. 2012;60(39):9882–9. doi: 10.1021/jf302527p. [DOI] [PubMed] [Google Scholar]
- 62.Sukardiman Sukardiman, Widyawaruyanti A, Widyowati R, Sismindari Sismindari, Zaini NC. Pinostrobin isolated from Kaempferia pandurata Roxb induced apoptosis in t47d human beast cancer cell line. E-Journal Planta Husada. 2014;2(1):20–6. [Google Scholar]
- 63.Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22(42):6549–56. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 64.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26(2):281–90. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 65.Yue GGL, Kwok HF, Lee JKM, Jiang L, Chan KM, Cheng L. et al. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J Funct Foods. 2015;15:243–53. doi: 10.1016/j.jff.2015.03.030. [DOI] [Google Scholar]
- 66.Shanmugam MK, Hsu A, Hui KM, Tan BKH, Sethi G. Abstract 4123: Thymoquinone inhibits bone metastasis in a breast cancer mouse model by modulating CXCR4/CXCL12 signaling axis. Cancer Res. 2016;76(14 Suppl):4123. doi: 10.1158/1538-7445.am2016-4123. [DOI] [Google Scholar]
- 67.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR. et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557–62. doi: 10.3892/or.15.6.1557. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Liu Y, Zou J, Yan L, Du W, Zhang Y. et al. Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1alpha and autophagy in human osteosarcoma. Oncotarget. 2017;8(53):91134–49. doi: 10.18632/oncotarget.19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 70.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 71.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 72.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 73.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 74.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23(2):97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 75.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):201–12. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao N, Li YH, Wu XK, Wang GY, Cai DY, Han FJ. [Effect of Brucea javanica fruit oil emulsion combined cisplatin on the growth inhibition of transplanted tumor in human ovarian cancer SKOV3 nude mice: an experimental study] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(1):57–62. [PubMed] [Google Scholar]
- 77.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA. et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9(5):1419–31. doi: 10.1158/1535-7163.mct-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jenie RI, Handayani S, Susidarti RA, Udin Z, Meiyanto E. Cytotoxic and Antimetastasis Effect of Ethyl Acetate Fraction from Caesalpinia sappan L on MCF-7/HER2 Cells. Indonesian Journal of Cancer Chemoprevention. 2017;8(1):42–50. doi: 10.14499/indonesianjcanchemoprev8iss1pp42-50. [DOI] [Google Scholar]
- 79.Hsieh CY, Tsai PC, Chu CL, Chang FR, Chang LS, Wu YC. et al. Brazilein suppresses migration and invasion of MDA-MB-231 breast cancer cells. Chem Biol Interact. 2013;204(2):105–15. doi: 10.1016/j.cbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Handayani S, Susidarti RA, Udin Z, Meiyanto E, Jenie RI. Brazilein in combination with cisplatin inhibit proliferation and migration on highly metastatic cancer cells, 4T1. Indonesian Journal of Biotechnology. 2016;21(1):38–47. doi: 10.22146/ijbiotech.26106. [DOI] [Google Scholar]
- 81.Kim BS. Brazilin inhibits of TPA-induced MMP-9 expression via the suppression of NF-κB activation in MCF-7 human breast carcinoma cells. Journal of Food Hygiene and Safety. 2010;25(3):209–14. [Google Scholar]
- 82.Lai SL, Cheah SC, Wong PF, Noor SM, Mustafa MR. In vitro and in vivo anti-angiogenic activities of Panduratin A. PLoS One. 2012;7(5):e38103. doi: 10.1371/journal.pone.0038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi MA, Kim SH, Chung WY, Hwang JK, Park KK. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem Biophys Res Commun. 2005;326(1):210–7. doi: 10.1016/j.bbrc.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 84.Yodkeeree S, Ampasavate C, Sung B, Aggarwal BB, Limtrakul P. Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur J Pharmacol. 2010;627(1-3):8–15. doi: 10.1016/j.ejphar.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 85.Chen CC, Sureshbabul M, Chen HW, Lin YS, Lee JY, Hong QS. et al. Curcumin suppresses metastasis via Sp-1, FAK inhibition, and E-cadherin upregulation in colorectal cancer. Evid Based Complement Alternat Med. 2013;2013:541695. doi: 10.1155/2013/541695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park SY, Kim YH, Kim Y, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-kappaB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012;113(12):3653–62. doi: 10.1002/jcb.24238. [DOI] [PubMed] [Google Scholar]
- 87.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E. et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35 Suppl:S185–s98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Gasparoto TH, de Souza Malaspina TS, Benevides L, de Melo EJ, Jr Jr, Costa MR, Damante JH. et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59(6):819–28. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71(22):6915–20. doi: 10.1158/0008-5472.can-11-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–18. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 91.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 92.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G. et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–71. doi: 10.1158/0008-5472.can-10-2907. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R. et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142(2):245–50. doi: 10.1016/j.jconrel.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 94.Majdalawieh AF, Hmaidan R, Carr RI. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. 2010;131(2):268–75. doi: 10.1016/j.jep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 95.Salem ML, Alenzi FQ, Attia WY. Thymoquinone, the active ingredient of Nigella sativa seeds, enhances survival and activity of antigen-specific CD8-positive T cells in vitro. Br J Biomed Sci. 2011;68(3):131–7. doi: 10.1080/09674845.2011.11730340. [DOI] [PubMed] [Google Scholar]
- 96.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S. et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7(4):306–15. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yonggang T, Yiming M, Heying Z, Cheng S, Qiushi W, Xianghong Y. et al. Maturation and upregulation of functions of murine dendritic cells (DCs) under the influence of purified aromatic-turmerone (AR) Hum Vaccin Immunother. 2012;8(10):1416–24. doi: 10.4161/hv.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 99.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 100.Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M. et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev. 2013;2013:387014. doi: 10.1155/2013/387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 102.Samadi AK, Bilsland A, Georgakilas AG, Amedei A, Amin A, Bishayee A. et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin Cancer Biol. 2015;35 Suppl:S151–s84. doi: 10.1016/j.semcancer.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 104.Goulet AC, Einsphar JG, Alberts DS, Beas A, Burk C, Bhattacharyya A. et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer Biol Ther. 2003;2(6):713–8. doi: 10.4161/cbt.2.6.627. [DOI] [PubMed] [Google Scholar]
- 105.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191(2):125–35. doi: 10.1016/S0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 106.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK. et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–68. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 107.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 109.Yun JM, Kwon H, Mukhtar H, Hwang JK. Induction of apoptosis by Panduratin A isolated from Kaempferia pandurata in human colon cancer HT-29 cells. Planta Med. 2005;71(6):501–7. doi: 10.1055/s-2005-864149. [DOI] [PubMed] [Google Scholar]
- 110.Bae IK, Min HY, Han AR, Seo EK, Lee SK. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in RAW 2647 macrophage cells. Eur J Pharmacol. 2005;513(3):237–42. doi: 10.1016/j.ejphar.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 111.Lou GG, Yao HP, Xie LP. Brucea javanica oil induces apoptosis in T24 bladder cancer cells via upregulation of caspase-3, caspase-9, and inhibition of NF-kappaB and COX-2 expressions. Am J Chin Med. 2010;38(3):613–24. doi: 10.1142/s0192415x10008093. [DOI] [PubMed] [Google Scholar]
- 112.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11(5):373–81. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee SK, Hong CH, Huh SK, Kim SS, Oh OJ, Min HY. et al. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J Environ Pathol Toxicol Oncol. 2002;21(2):141–8. doi: 10.1615/JEnvironPatholToxicolOncol.v21.i2.70. [DOI] [PubMed] [Google Scholar]
- 114.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 115.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 118.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27(2):211–22. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Fath MA, Scarbrough PM, Watson WH, Spitz DR. Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biol. 2015;4:127–35. doi: 10.1016/j.redox.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oh ET, Kim CW, Kim HG, Lee JS, Park HJ. Brusatol-mediated inhibition of c-Myc increases HIF-1α degradation and causes cell death in colorectal cancer under hypoxia. Theranostics. 2017;7(14):3415–31. doi: 10.7150/thno.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gunnink LK, Alabi OD, Kuiper BD, Gunnink SM, Schuiteman SJ, Strohbehn LE. et al. Curcumin directly inhibits the transport activity of GLUT1. Biochimie. 2016;125:179–85. doi: 10.1016/j.biochi.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lau ST, Lin ZX, Leung PS. Role of reactive oxygen species in brucein D-mediated p38-mitogen-activated protein kinase and nuclear factor-kappaB signalling pathways in human pancreatic adenocarcinoma cells. Br J Cancer. 2010;102(3):583–93. doi: 10.1038/sj.bjc.6605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai ZQ, Ip SP, Liao HJ, Lu Z, Xie JH, Su ZR. et al. Brucein D, a Naturally Occurring Tetracyclic Triterpene Quassinoid, Induces Apoptosis in Pancreatic Cancer through ROS-Associated PI3K/Akt Signaling Pathway. Front Pharmacol. 2017;8:936. doi: 10.3389/fphar.2017.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim JY, An JM, Chung WY, Park KK, Hwang JK, Kim du S. et al. Xanthorrhizol induces apoptosis through ROS-mediated MAPK activation in human oral squamous cell carcinoma cells and inhibits DMBA-induced oral carcinogenesis in hamsters. Phytother Res. 2013;27(4):493–8. doi: 10.1002/ptr.4746. [DOI] [PubMed] [Google Scholar]
- 125.Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep. 2018;8(1):2039. doi: 10.1038/s41598-018-20179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. O’Neil MJ. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Cambridge, UK: Royal Society of Chemistry; 2013.
- 127.Salmani JM, Asghar S, Lv H, Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light . Molecules. 2014;19(5):5925–39. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Block KI, Gyllenhaal C, Lowe L, Amedei A, Amin A, Amin A. et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin Cancer Biol. 2015;35 Suppl:S276–s304. doi: 10.1016/j.semcancer.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


