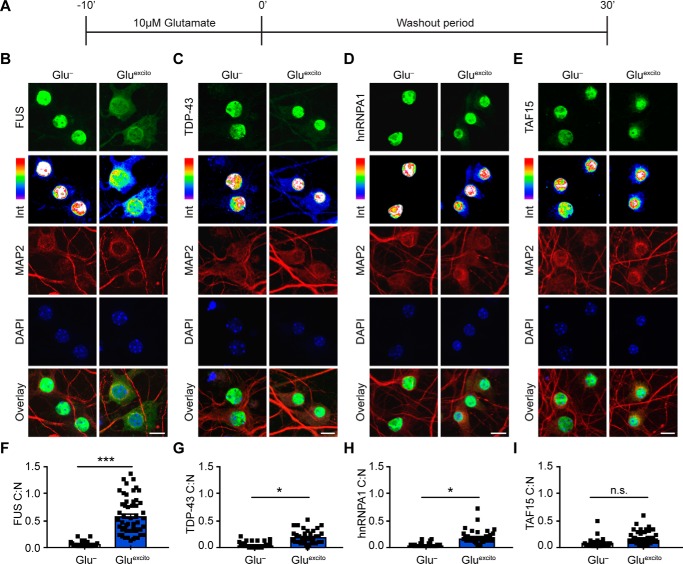
Figure 1.
Endogenous FUS robustly translocates to the cytoplasm in response to excitotoxic stress. A, DIV 14–16 primary cortical neurons were bath-treated with 10 μm glutamate (Gluexcito) for 10 min, after which the glutamate-containing media was “washed out” and replaced with cultured neuronal media for an additional 30 min. B–E, immunofluorescence and confocal microscopy revealed the cellular localization of FUS, TDP-43, hnRNPA1, and TAF15 (green) in the absence and presence of Gluexcito. Endogenous RBP staining (green) visualized by a 16-color intensity map (Int) further demonstrates the cytoplasmic presence of these proteins. Neurons and dendrites were identified with anti-MAP2 staining (red), and nuclei with DAPI (blue). Scale bars = 10 μm. F–I, quantification of the cytoplasmic to nuclear ratio (C:N) from B–E was based on fluorescence intensities of the signal in each compartment as described under “Experimental procedures.” A significant nuclear egress of FUS (F), TDP-43 (G), and hnRNPA1 (H) but not TAF15 (I) was observed following Gluexcito treatment (n = 3–4 biological replicates). Black squares represent the C:N ratio of individual cells, and error bars correspond to S.E. Experimental means were calculated from the average C:N ratio across the individual biological replicates and significant comparisons were determined with a Student's t test (FUS: **, p = 0.0013; hnRNPA1: *, p = 0.0107; TDP-43: *, p = 0.0185; n.s., nonsignificant).