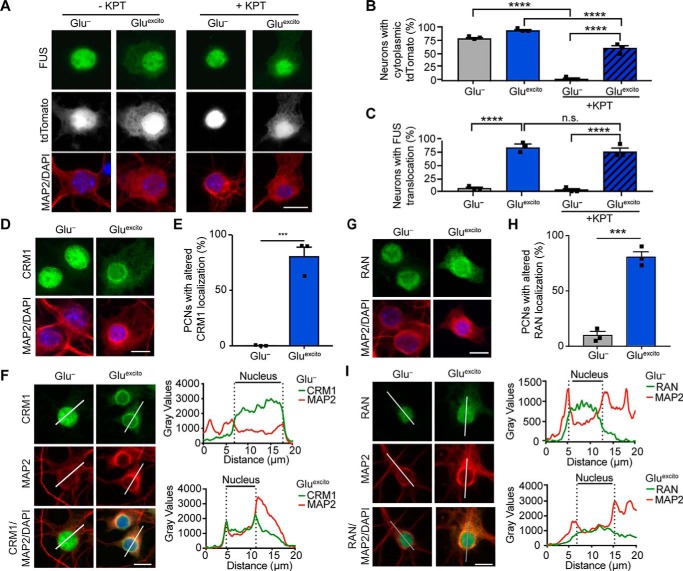
Figure 4.
Nucleocytoplasmic transport is disrupted by Gluexcito. A–C, cortical neurons expressing the shuttling reporter, NLS–tdTomato–NES, were treated with or without 500 nm of the exportin 1 inhibitor KPT-330 (KPT) prior to Gluexcito exposure. Neurons were identified with anti-MAP2 staining (A; red). The percentage of MAP2-positive cells expressing cytoplasmic NLS–tdTomato–NES (A; white) or FUS (A; green) was quantified in B and C, respectively (n = 3 biological experiments). KPT-330 effectively prevents NLS–tdTomato–NES from localizing to the cytoplasm in the absence of stress (Glu−; two-way ANOVA and Tukey's post hoc test; for all statistical comparisons, ****, p < 0.0001), as expected. Conversely, in the presence of stress (Gluexcito), KPT-330 fails to restrict NLS–tdTomato–NES and FUS localization to the nucleus, indicative of dysregulated nucleocytoplasmic transport (B and C, compare Glu− to Gluexcito in the presence of KPT-330, two-way ANOVA, and Tukey's post hoc test; for all significant statistical comparisons, ****, p < 0.0001, n.s. = nonsignificant). The localization of nuclear transport factors CRM1 (D–F) and RAN (G–I) were significantly altered under conditions of Gluexcito in MAP2-positive neurons (red); cytoplasmic CRM1 and RAN (green in D and E, respectively) were more apparent in stressed cells. The percentage of neurons with CRM1 or RAN mislocalization was quantified in E and H, respectively (Student's t test; CRM1, ***, p = 0.0006; RAN, ***, p = 0.0003; n = 3 biological replicates). Error bars represent S.E. F–I, representative line scan analyses of CRM1 and RAN staining demonstrates an increase in the cytoplasmic presence of these proteins. Specific to CRM1, an increase in staining intensity at the nuclear periphery was observed in neurons exposed to excitotoxic insult. Scale bars = 10 μm.