Figure 7.
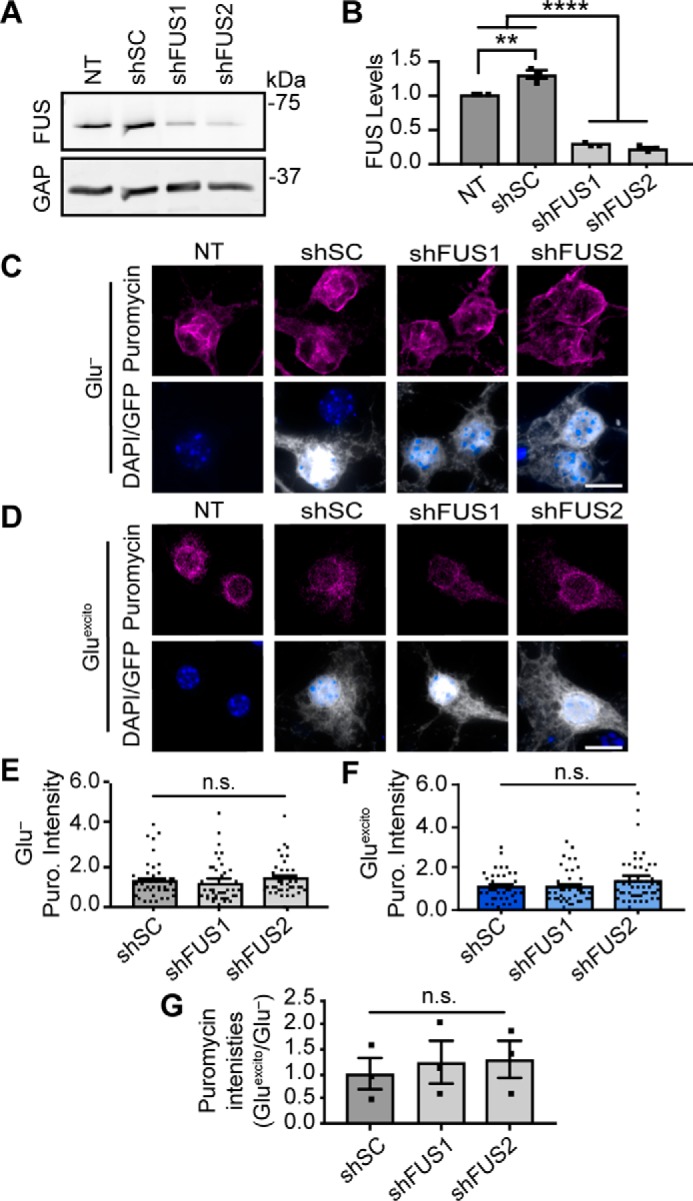
Reduced protein translation following excitotoxic stress is independent of FUS levels. Primary neurons were transduced with shRNAs against mouse FUS (shFUS1 and shFUS2) or a scrambled control (scrambled control) to induce FUS knockdown. A and B, Western and densitometry analysis confirms FUS knockdown relative to nontransduced (NT) and shSC conditions. A modest increase in FUS levels was observed upon expression of shSC relative the loading standard, GAPDH (GAP; n = 3; one-way ANOVA and Tukey's post hoc test, ****, p < 0.0001; **, p = 0.0020; n = 3 biological replicates). C and D, neurons were pulse-chased labeled with puromycin (Puro; magenta) to assess nascent protein translation in transduced cells (as in B–D). The intensity of puromycin (Puro) staining for each condition was normalized to the respective stressed or unstressed nontransduced (NT) control. Scale bar = 10 μm. E and F, quantification of puromycin (Puro) staining from C and D reveals no statistically significant difference in the somatic levels of translation following FUS knockdown (shFUS1 and shFUS2) relative to shSC (one-way ANOVA and Dunnett's post hoc test, n.s. = not significant, n = 3 biological replicates). G, furthermore, there was no significant difference in the relative amount of puromycin intensity between stress and nonstress conditions (Gluexcito/Glu−) as a result of FUS levels (shSC, shFUS1, and shFUS2). For stress and nonstress conditions, puromycin intensities were normalized to the respective, nontransduced control; black squares represent the means represent from n = 3 biological replicates (one-way ANOVA and Tukey's post hoc test, n.s. = nonsignificant). Error bars = S.E.
