Figure 8.
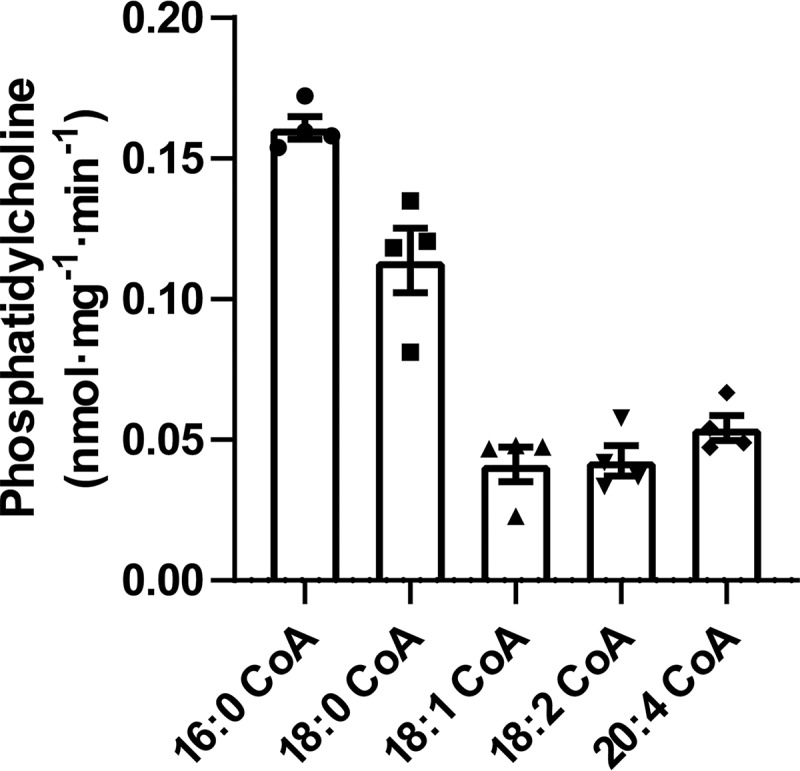
Substrate specificities of the microsomal sn-1 acyltransferase reaction. Microsomal homogenates isolated from mouse liver were incubated with 10 μm l-2-AA-ether-LPC in the presence of selected saturated and unsaturated molecular species of acyl-CoAs for 5 min at 37 °C in 75 mm sodium phosphate buffer (pH 7.4). The reactions were terminated by addition of chloroform/methanol (1:1, v/v) and vortexed. The chloroform layer was isolated and dried under a nitrogen stream. The dried residues were redissolved in water/methanol (1:4), and the sn-1 acyltransferase product, 1-acyl-2-AA-ether-PC, was analyzed and quantitated by LC-MS in the positive ion mode. Values are the average of four independent preparations. Error bars represent S.D.
