Key Points
Question
Do patients with large stroke with substantial ischemic changes on imaging achieve reasonable functional and safety outcomes with thrombectomy compared with medical management only?
Findings
This prespecified secondary analysis of a cohort study analyzed 105 patients with substantial ischemic changes on computed tomographic or computed tomographic perfusion imaging, of whom 62 received endovascular thrombectomy. Functional independence was achieved in 31% of patients who received endovascular thrombectomy vs 14% who received medical management only, while deaths, neurological worsening, and symptomatic intracerebral hemorrhage were observed in similar proportions in both groups; also, the likelihood of functional independence declined by 40% with each hour delay and 42% with each 10-cm3 increase in stroke volume.
Meaning
Endovascular thrombectomy may result in therapeutic benefits for patients with large infarcts, especially if they are treated early and have a core volume less than 100 cm3.
This prespecified secondary analysis of a cohort study compares outcomes in patients with large ischemic cores treated with endovascular thrombectomy vs medical management alone.
Abstract
Importance
The efficacy and safety of endovascular thrombectomy (EVT) in patients with large ischemic cores remains unknown, to our knowledge.
Objective
To compare outcomes in patients with large ischemic cores treated with EVT and medical management vs medical management alone.
Design, Setting, and Participants
This prespecified analysis of the Optimizing Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) trial, a prospective cohort study of imaging selection that was conducted in 9 US comprehensive stroke centers, enrolled patients between January 2016 and February 2018, and followed them up for 90 days. Patients with moderate to severe stroke and anterior circulation large-vessel occlusion presenting up to 24 hours from the time they were last known to be well were eligible for the cohort. Of these, patients with large ischemic cores on computed tomography (CT) (Alberta Stroke Program Early CT Score <6) or CT perfusion scanning (a volume with a relative cerebral blood flow <30% of ≥50 cm3) were included in analyses.
Exposures
Endovascular thrombectomy with medical management (MM) or MM only.
Main Outcomes and Measures
Functional outcomes at 90 days per modified Rankin scale; safety outcomes (mortality, symptomatic intracerebral hemorrhage, and neurological worsening).
Results
A total of 105 patients with large ischemic cores on either CT or CT perfusion images were included: 71 with Alberta Stroke Program Early CT Scores of 5 or less (EVT, 37; MM, 34), 74 with cores of 50 cm3 or greater on CT perfusion images (EVT, 39; MM, 35), and 40 who had large cores on both CT and CT perfusion images (EVT, 14; MM, 26). The median (interquartile range) age was 66 (60-75) years; 45 patients (43%) were female. Nineteen of 62 patients (31%) who were treated with EVT achieved functional independence (modified Rankin Scale scores, 0-2) vs 6 of 43 patients (14%) treated with MM only (odds ratio [OR], 3.27 [95% CI, 1.11-9.62]; P = .03). Also, EVT was associated with better functional outcomes (common OR, 2.12 [95% CI, 1.05-4.31]; P = .04), less infarct growth (44 vs 98 mL; P = .006), and smaller final infarct volume (97 vs 190 mL; P = .001) than MM. In the odds of functional independence, there was a 42% reduction per 10-cm3 increase in core volume (adjusted OR, 0.58 [95% CI, 0.39-0.87]; P = .007) and a 40% reduction per hour of treatment delay (adjusted OR, 0.60 [95% CI, 0.36-0.99]; P = .045). Of 10 patients who had EVT with core volumes greater than 100 cm3, none had a favorable outcome.
Conclusions and Relevance
Although the odds of good outcomes for patients with large cores who receive EVT markedly decline with increasing core size and time to treatment, these data suggest potential benefits. Randomized clinical trials are needed.
Introduction
Endovascular thrombectomy is safe and efficacious up to 24 hours after stroke onset in selected patients with acute ischemic strokes caused by large-vessel occlusion in the anterior circulation.1,2,3,4,5,6,7 The clinical trials evaluating thrombectomy inclusion criteria have focused on patients with minimal ischemic changes on imaging prior to thrombectomy on noncontrast computed tomography (CT) (Alberta Stroke Program Early CT Score [ASPECTS], ≥6)2,3,4 or small ischemic cores (a volume with a relative cerebral blood flow <30% of <50 cm3 or <70 cm3 on computed tomographic perfusion [CTP] images).5,6,7 These criteria have resulted in largely excluding patients with large ischemic cores at baseline.
Patients with larger ischemic changes on noncontrast CT or CTP are less likely to achieve high rates of functional independence, given that substantial stroke evolution already has taken place and irreversible damage has occurred. Moreover, there are safety concerns; reperfusion of large infarcted areas carries a higher risk of hemorrhagic transformation. Thus, the risk-benefit ratio for thrombectomy is not well established in the subpopulation with large cores or low ASPECTS.
Furthermore, the definition of a large ischemic core differs between CT and perfusion imaging, given the difference of the 2 imaging modalities in the assessment of brain ischemia. While a CT scan that incorporates the ASPECTS detects hypodense tissue, perfusion imaging identifies regions of very low blood flow or volume. This difference could affect treatment decisions and result in a variability of thrombectomy clinical outcomes.
We describe and compare the clinical outcomes and safety of endovascular thrombectomy in patients with large ischemic infarct on CT or CTP images compared with medical management alone. We also assess the variability of endovascular thrombectomy outcomes in association with the volume of the early ischemic lesion and time to thrombectomy treatment.
Methods
The study was a prespecified subanalysis of the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT): a Prospective Multicenter Cohort Study of Imaging Selection (NCT02446587). The SELECT trial enrolled patients with stroke attributable to large-vessel occlusion who were treated with endovascular thrombectomy plus medical management (termed EVT herein) or medical management alone, based on CT or CTP findings, up to 24 hours from the point that they were last known to be well.
All patients received noncontrast CT, CT angiography, and CTP using RAPID software (iSchemaView) for automated mismatch determination, as well as a follow-up magnetic resonance image (MRI) or noncontrast CT more than 24 to 36 hours after enrollment. An independent core laboratory (headed by C.S.) at University of Texas McGovern Medical School, blinded to clinical outcomes, site, and imaging selection modality, evaluated all available imaging. Modified Rankin Scale (mRS) score assessment was performed at 90 days by assessors blinded to the core laboratory reading and treatment assignment (EVT vs medical management).
The CTP protocols for each site were adjusted to harmonize acquisition parameters. The criteria were brain coverage of at least 8 cm on the z-axis, temporal sampling resolution of no more than 1.8 seconds, a tube voltage of 580-kV peak, a volume CT dose index of less than 360 mGy, a scan duration between 60 and 90 seconds, a contrast agent with a high iodine concentration (eg, iohexol or iopamidol), an injection flow rate between 4 and 6 mL/s, and an amount of contrast injected between 40 and 50 mL, with no scan delay after bolus injection. Allowed scan modes used were burst mode (GE, Toshiba, Philips), jog mode (Philips), or dynamic helical shuttle (Siemens). For CT equipment with a detector width less than 8 cm, either 2 CTP runs or the use of dynamic helical shuttle mode were required. Iterative reconstruction methods were avoided to reduce variability between vendors.
The study protocol was approved by the institution review board at each participating site prior to the start of the study. Written informed consent was obtained from the patient or his or her guardian or legal representative prior to data entry into the SELECT database and up to 7 days postprocedure.
Study Population
Patients with large cores were defined as having an ASPECTS of 5 or less on noncontrast CT or an ischemic core volume of 50 cm3 or more on CTP based on the volume of tissue with a relative cerebral blood flow less than 30% at presentation. Patients were divided based on the treatment received into a group receiving EVT vs a group receiving medical management alone. The decision to proceed with EVT vs medical management alone was made at the discretion of the local investigators in a nonrandomized fashion. The study initially included patients up to 8 hours after stroke onset, with the enrollment window extended up to 24 hours after the results of the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial6 were announced.
Study Treatments and Interventions
Mechanical thrombectomy primarily included the use of stent retrievers or other US Food and Drug Administration–approved devices as recommended by the American Heart Association guidelines.8 Adjunctive use of aspiration was allowed. Use of intra-arterial lytics and stenting was allowed as per individual site treatment protocols.
Each study site was allowed to use their standard thrombectomy procedure practices for femoral access, sedation, monitoring, and other tasks. Standard American Heart Association guideline–directed medical management was provided to all patients.
Study Outcomes
The primary outcome was the 90-day mRS score. A good outcome was defined as functional independence (mRS scores, 0-2). Good to moderate functional dependence was defined as an mRS score of 0 to 3, since the mRS score of 3 is considered a reasonable outcome in patients with large ischemic cores and substantial ischemic changes on imaging. Safety outcomes included symptomatic intracerebral hemorrhage (worsening of National Institutes of Health Stroke Scale [NIHSS] score of 4 or more, with evidence of any intracerebral hemorrhage on follow-up imaging),9 neurological worsening (a worsening of the NIHSS score of 4 or more at 24 hours after the presentation NIHSS score was recorded), and mortality at 90 days. Imaging outcomes for patients treated with EVT were postprocedure modified treatment in cerebral ischemia scores (with a successful reperfusion defined by a score of ≥2b) and final infarct volumes measured on follow-up MRI diffusion-weighted images or CT scans when MRIs were not available.
Statistical Analysis
Demographic factors, medical history, and baseline characteristics of the patient cohort were described and compared between patients who received EVT and those who received medical management only. Characteristics were compared by 2-sample t test or Wilcoxon rank sum test for continuous variables and χ2 test or Fisher exact test for categorical variables.
First, the clinical outcomes were compared between the groups receiving EVT vs medical management only. A shift analysis of mRS scores (of 0-6 points) based on the proportional odds model was performed using ordinal logistic regression model, adjusting for the potential confounders of age, NIHSS scores at presentation, baseline core volume, serum glucose at presentation, clot location, intravenous tissue plasminogen activator status, and time. Patients with mRS scores of 0 and 1 were combined for shift analysis because of the limited sample size. The adjusted common odds ratios (aORs) of shifting from 1 category to the next were reported with 95% CIs. Furthermore, a comparison of 90-day functional independence rates was also performed to compare the EVT and medical management groups, after adjusting for the same baseline variables using logistic regression model. The adjusted odds ratios (aORs; with 95% CIs) of having functional independence were reported. Additional detailed analyses of patients with large ischemic cores on CT (ASPECTS <6), CTP (a volume with a relative cerebral blood flow <30% of ≥50 cm3), and both imaging modalities were also reported.
Second, in patients treated with EVT, we assessed the variables independently associated with 90-day functional independence through a multivariable logistic regression model. Clinically important variables (eg, intravenous tissue plasminogen activator status and clot location), as well as variables with P < .10 in a univariable analysis assessing their association with good outcomes, were entered into the model. A stepwise method was used for variable selection. The aORs (with 95% CIs) of functional independence were reported.
We further assessed the outcomes associated with the different volume thresholds on CTP as well as ASPECTS on the outcomes of EVT. A sensitivity analysis was performed to assess for changes in the results in patients with an ischemic core volume cutoff of more than 70 cm3. Because prior EVT clinical trials3 have used ASPECTS score of 6 as the cutoff for imaging exclusion criteria, a sensitivity analysis of outcomes in patients with ASPECTS score of 6 or less was also performed (eTable 6 in the Supplement).
Stata version 15 (StataCorp; 2017) and SAS version 9.4 (SAS Institute) were used to perform data analyses. All reported P values are 2-sided, and P values less than .05 were considered statistically significant.
Results
Patients Cohort and Baseline Characteristics
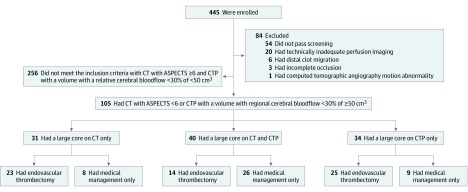
Of 4722 screened patients in the SELECT study between January 2016 and February 2018, 445 were eligible and consented; 84 did not meet study criteria and were excluded from analyses. Of the 361 remaining individuals, 105 (29.1%) were classified as having large cores on CT (ASPECTS, 0-5) or CTP (ischemic core volume, ≥50 cm3) at the time of presentation. Sixty-two patients received medical management plus EVT, while 43 had medical management alone. Forty patients (EVT, 14; medical management, 26) had demonstrated large ischemic cores on both CT and CTP, whereas 31 had large ischemic cores on CT alone, and 34 patients had large ischemic cores on CTP alone. Figure 1 shows a flowchart of patients enrolled in the SELECT trial, including patients with large ischemic cores and their distribution, based on the treatment received and imaging profile. Of note, 53 patients had an ischemic core volume greater than 70 cm3 (EVT, 24; medical management, 29).
Figure 1. Flowchart of Patients Enrolled in the Optimizing Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) Study, Patients With Large Cores, and Distribution Based on Treatment and Imaging Profile.
The number enrolled refers only to the 445 individuals who were eligible after screening and consented to participate. ASPECTS indicates Alberta Stroke Program Early CT Score; CT, computed tomography; and CTP, computed tomographic perfusion (imaging).
The demographics, medical history, baseline, imaging characteristics, and procedural characteristics of the patients were described and compared based on the treatment received (EVT and medical management) (Table 1). Key differences between the 2 groups were the time elapsed from the point when the patient was last seen well to arrival to the enrollment site, the size of the baseline ischemic core volume, and the rate of transferred patients to EVT-capable centers. All patients receiving EVT and all but 1 patient receiving medical management had a mismatch of more than 20% between the ischemic core volume and the lesion on CTP, with a mismatch volume (eg, the volume of the brain tissue with a time-to-maximum (T-max) value >6 seconds but a relative cerebral blood flow of >30%) of 10 cm3 or more. The median (interquartile range) mismatch volume was 121 (78-168) cm3 in patients treated with EVT and 95 (70-144) cm3 in those treated with medical management.
Table 1. Baseline Characteristics of the Cohort Based on Treatment Received.
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| Endovascular Thrombectomy (n = 62) | Medical Management Only (n = 43) | ||
| Age, median (IQR), y | 66 (59-74) | 66 (60-81) | .54a |
| Female | 25 (40) | 20 (47) | .53b |
| Serum glucose, median (IQR), mg/dL | 130 (114-177) | 131 (113-158) | .76a |
| Hypertension | 49 (80) | 34 (79) | .88b |
| Congestive heart failure | 8 (13) | 5 (12) | .80b |
| Coronary artery disease | 11 (18) | 8 (19) | .94b |
| Atrial fibrillation | 14 (23) | 10 (24) | .92b |
| Diabetes | 23 (38) | 15 (35) | .77b |
| Prior transient ischemic attack | 3 (5) | 8 (19) | .05c |
| Prior stroke | 8 (13) | 3 (7) | .35c |
| Smoker | |||
| Current | 6 (11) | 11 (26) | .04b |
| Past | 11 (19) | 4 (10) | .18c |
| Blood pressure at presentation, median (IQR), mm Hg | |||
| Systolic | 139 (125-162) | 150 (130-163) | .19a |
| Diastolic | 80 (72-87) | 81 (66-90) | .92a |
| Clot location | |||
| Internal carotid artery | 19 (31) | 16 (37) | .36b |
| Middle cerebral artery | |||
| M1 segment | 37 (60) | 20 (47) | |
| M2 segment | 6 (10) | 7 (16) | |
| Transferred to endovascular thrombectomy–capable center | 27 (44) | 30 (70) | .01b |
| Arrival to endovascular thrombectomy–capable hospital, median (IQR), min | 135 (58-256) | 246 (153-351) | .001a |
| Intravenous tissue plasminogen activator given | 43 (69) | 26 (60) | .35b |
| National Institutes of Health Stroke Scale score at presentation, median (IQR) | 20 (16-23) | 21 (17-23) | .56a |
| Door to computed tomographic imaging time, median (IQR) | 12 (2-20) | 9 (0-20) | .58a |
| Door to computed tomographic perfusion imaging time, median (IQR) | 20 (13-29) | 15 (6-27) | .06a |
| Time from last seen well to endovascular thrombectomy, median (IQR), min | 223.5 (152-339) | NA | NA |
| Alberta Stroke Program Early CT Score, median (IQR) | 5 (4-7) | 4 (3-5) | <.001a |
| Volume with a relative cerebral blood flow <30%, median (IQR) | 60.0 (37.0-87.4) | 86.6 (64.7-118.0) | .002a |
| Mismatch volume, median (IQR) | 120.9 (78.0-168.0) | 95.0 (70.1-144.0) | .16a |
| Passes, No. | |||
| Multiple | 32 (52) | NA | NA |
| Single | 29 (48) | NA | |
| Anesthesia | |||
| Conscious sedation | 36 (58) | NA | NA |
| General anesthesia | 26 (42) | NA | |
Abbreviations: CT, computed tomography; IQR, interquartile range; NA, not applicable.
SI conversion factor: To convert serum glucose to millimoles per liter, multiply by 0.0555.
P value obtained using Wilcoxon rank sum test.
P value obtained using Pearson χ2 test.
P value obtained using Fisher exact test.
Outcomes of EVT vs Medical Management
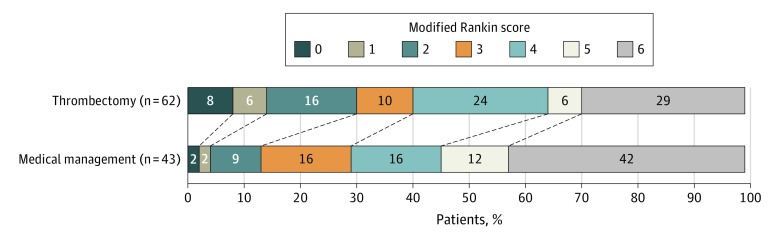
There were higher rates of functional independence at 90 days in the EVT group (19 of 62 [31%]) compared with those treated only with medical management (6 of 43 [14%]; OR, 3.27 [95% CI, 1.11-9.62]; P = .03; Table 2), and there was also a shift toward better outcomes across the mRS categories with EVT (common OR, 2.12 [95% CI, 1.05-4.31]; P = .04; Figure 2). Good to moderate outcomes (mRS scores, 0-3) were numerically more common (EVT, 25 of 62 [40%] vs medical management, 13 of 43 [30%]), but the difference did not reach statistical significance (P = .29). After adjustment for potential confounders, there was a nonsignificant finding of better outcome with EVT (patients with mRS scores of 0 to 2: aOR, 3.95 [95% CI, 0.62-25.35]; P = .15; eTable 1 in the Supplement; shift analysis: common OR, 1.61 [95% CI, 0.71-3.63]; P = .25; eTable 2 in the Supplement). In regard to imaging outcomes, patients treated with EVT compared with those who received medical management only had smaller final infarct volumes (median [interquartile range], 97 (43-189) mL vs 190 (127-252) mL; P < .001) and less infarct growth (44 [0.7-107.6] mL vs 98 [63-149] mL; P = .006). There was a numerically higher but not significantly different rate of symptomatic intracerebral hemorrhage (ICH) with EVT (EVT, 8 of 62 [13%] vs medical management, 3 of 43 [7%]; P = .51) but no difference in neurological worsening (EVT, 13 of 62 [21%] vs medical management, 8 of 43 [20%]; P = .87) or mortality with EVT (18 of 62 [29%]) compared with medical management (8 of 63 [42%]; P = .17).
Table 2. Clinical and Radiographic Outcomes of the Cohort Based on Treatment Received.
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| Endovascular Thrombectomy (n = 62) | Medical Management Only (n = 43) | ||
| 90-d modified Rankin Scale score | |||
| 0 | 5 (8) | 1 (2) | .03a |
| 1 | 4 (6) | 1 (2) | |
| 2 | 10 (16) | 4 (9) | |
| 3 | 6 (10) | 7 (16) | |
| 4 | 15 (24) | 7 (16) | |
| 5 | 4 (6) | 5 (12) | |
| 6 | 18 (29) | 18 (42) | |
| Good outcomes | 19 (31) | 6 (14) | .05b |
| Good to moderate outcomesc | 25 (40) | 13 (30) | .29b |
| Neurological worsening | 13 (21) | 8 (20) | .87b |
| Symptomatic intracranial hemorrhage | 8 (13) | 3 (7) | .51d |
| Any intracranial hemorrhage | 37 (60) | 15 (36) | .02b |
| Death | 18 (29) | 18 (42) | .17b |
| Successful reperfusione | 50 (81) | NA | NA |
| Final infarct volume, median (IQR), cm3 | 97 (43-189) | 190 (127-252) | .001f |
| Infarct growth, median (IQR), cm3 | 44 (1-108) | 98 (63-149) | .006f |
Abbreviation: NA, not applicable.
P value obtained using univariate ordinal logistic regression.
P value obtained using the Pearson χ2 test.
Defined as modified Rankin Scale scores of 0 to 3.
P value obtained using the Fisher exact test.
Modified treatment in cerebral ischemia grade of 2b or greater.
P value obtained using Wilcoxon rank sum test.
Figure 2. Ninety-Day Modified Rankin Scale Score Distribution in Patients Treated With Endovascular Thrombectomy vs Medical Management Only.
Percentages do not add up to 100% owing to rounding.
Outcomes by Imaging Modality
Large Cores on CT
Of 71 patients with large cores on CT, 37 (52%) received EVT, and 34 (48%) were treated with medical management only. Functional independence rates were numerically but nonsignificantly higher in patients treated with EVT (13 [35%]) compared with medical management only (6 [18%]; P = .10; eFigure 1 in the Supplement). There was also a shift toward better outcomes across the mRS categories with EVT, which did not reach statistical significance (adjusted common OR, 1.76 [95% CI, 0.67-4.62]; P = .25). eTable 3 in the Supplement displays the unadjusted and adjusted logistic and ordinal logistic regression models for EVT outcomes on functional independence and 90-day mRS score distribution in patients with large cores by imaging modality. Rates of safety outcomes, namely symptomatic ICH (4 [11%] vs 3 [9%]; P > .99) and neurological worsening (7 [19%] vs 4 [13%]; P = .53), were similar between the EVT and medical management arms. Mortality rates (EVT, 6 [16%] vs medical management, 13 [38%]; P = .06) did not reach statistical significance. Similar results were observed in a sensitivity analysis in patients with ASPECTS of 6 or less (eTable 6 in the Supplement).
Large Cores on CTP
Of 74 patients with large cores on CTP, 39 (53%) received EVT, and 35 (47%) received medical management only. Rates of functional independence (8 [21%] vs 1 [3%]; P = .03) were significantly higher in patients treated with EVT (eFigure 1 in the Supplement) There was a numerical difference in functional outcome that was not statistically significant (adjusted common OR, 1.53 [95% CI, 0.54-4.30]; P = .42; eTable 3 in the Supplement). Rates of symptomatic ICH (7 [18%] vs 3 [9%]; P = .24) and neurological worsening (12 [32%] vs 7 [21%]; P = .33) were numerically but nonsignificantly higher in patients treated with EVT. Mortality rates (17 [44%] vs 17 [49%]; P = .67) were similar between patients who received EVT vs those who received medical management.
Large Cores on CT and CTP
Forty patients had large cores on both imaging modalities. Of these, 14 (35%) were treated with EVT, and 26 (65%) were treated with medical management only. Functional independence rates were found in 2 individuals (14%) in the EVT group compared with 1 individual (4%) in the medical management group (P = .28; eFigure 1 in the Supplement). Multivariable ordinal logistic regression model showed a nonsignificant difference in 90-day mRS scores (adjusted common OR, 3.24 [95% CI, 0.71-14.72]; P = .13; eTable 3 in the Supplement). Patients treated with EVT had significantly higher rates of neurological worsening (6 [46%] vs 3 [13%]; P = .04). Symptomatic ICH (3 [21%] vs 3 [12%]; P = .65) and deaths (5 [36%] vs 12 [46%]; P = .74) were similar between patients treated with EVT vs medical management only.
Outcomes Associated With Reperfusion
The successful reperfusion rate with EVT (modified treatment in cerebral ischemia ≥2b) was found in 50 of 62 individuals (81%). Patients who achieved successful reperfusion had numerically higher rates of functional independence (17 of 50 [34%] vs 2 of 12 [17%] in those who had modified treatment in cerebral ischemia scores of 0 to 2a), but this difference did not reach statistical significance (aOR, 2.50 [95% CI, 0.15-41.22]; P = .52; eFigure 2 in the Supplement).
Outcomes Associated With Stroke Size on CTP and Noncontrast CT
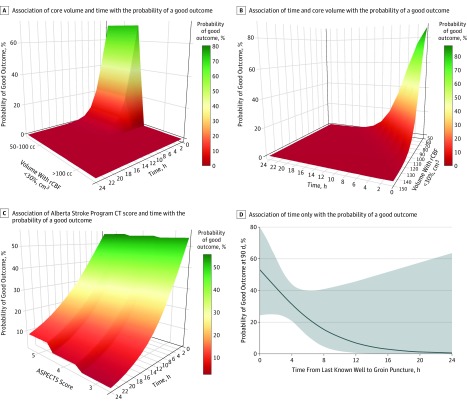
Patients with ischemic core volumes of 50 to 100 cm3 had good outcomes rates (8 of 29 [28%]), whereas no patient (0 of 10) with a core volume greater than 100 cm3 had functional independence at 90 days (eFigure 3 in the Supplement). The odds of a good outcome declined by 42% for each 10-cm3 increase in stroke volume on CTP (aOR, 0.58 [95% CI, 0.39-0.87]; P = .007). Similarly, rates of the safety outcomes increased, albeit nonsignificantly, with increasing core volume for patients with core volumes of 50 to 100 cm3 vs greater than 100 cm3 (symptomatic ICH, 3 of 29 [10%] vs 4 of 10 [40%]; P = .06; neurological worsening, 7 of 29 [25%] vs 5 of 10 [50%]; P = .24; and mortality, 10 of 29 [34%] vs 7 of 10 [70%]; P = .05). Figure 3A and B illustrate the outcomes of volume and time on the probability of 90-day functional independence with EVT.
Figure 3. Association of Time and Stroke Size With Thrombectomy Outcomes in Patients With Large Core on Computed Tomographic and Computed Tomographic Perfusion Images.
A, Association of volume (with a relative cerebral blood flow [rCBF] <30%) and time with the probability of a good outcome (90-day modified Rankin Scale score, 0-2) with endovascular thrombectomy (with ischemic core volume in categories of 50-100 cm3 or >100 cm3). A good outcome is associated with core volume and time of computed tomographic (CT) perfusion imaging. There is a decrease in likelihood of a good outcome with increase in the volume and as the time progresses. B, Association of volume (with rCBF <30%) and time with the probability of good outcome with endovascular thrombectomy (ischemic core volume as a continuous variable). A good outcome is associated with the core volume and time of CT perfusion imaging. There is a decrease in the likelihood of a good outcome with increases in volume and time progression. C, Association of Alberta Stroke Program Early CT Score (ASPECTS) and time with the probability of good outcome (90-day modified Rankin Scale score, 0-2) with endovascular thrombectomy (an ASPECTS of 3, 4, or 5). The probability of good outcome is associated with time but does not differ significantly with ASPECTS. There is a decrease in the likelihood of a good outcome with decrease in the ASPECTS and time progression. D, Association of time only on the probability of good outcome with endovascular thrombectomy in patients with large core. The probability of a good outcome decreases significantly as time progresses, with very low probability (<10%) after 12 hours.
The functional independence rates in patients receiving EVT were 40% (8 of 20) for those with ASPECTS of 5, 40% (4 of 10) for those with ASPECTS of 4, and 14% (1 of 7) for those with ASPECTS of 3. Of 34 patients with ASPECTS between 0 and 5 who were treated with medical management only, 2 of 11 patients (18%) with ASPECTS of 5, 3 of 6 (50%) with ASPECTS of 4, and 1 of 10 (10%) with ASPECTS of 3 achieved functional independence. No patient with ASPECTS of 0 to 2 achieved functional independence at 90 days, although all received medical management (eTable 4 in the Supplement). Restricting the comparison to patients with ASPECTS of 3 to 5, functional independence rates were numerically but nonsignificantly higher in patients treated with EVT (13 of 37 [35%] vs 6 of 27 [22%]; P = .26).
Figure 3C illustrates how the outcome of time to procedure changed with each ASPECTS increment. The interaction between ASPECTS and time to procedure on functional independence was not statistically significant. Good outcome rates numerically declined as the ASPECT decreased (a 14% reduction for each point reduction in ASPECTS), but these differences were not significant (aOR, 0.86 [95% CI, 0.54-1.37]; P = .52; eFigure 4 in the Supplement).
Sensitivity Analysis for Ischemic Core Volume Greater Than 70 cm3
To assess 2 different ischemic core volume cutoffs (>70 cm3 and >50 cm3), a sensitivity analysis was performed to evaluate the likelihood of a shift toward better EVT outcomes in patients with an ischemic core volume greater than 70 cm3 vs 50 to 70 cm3. Both groups had a similar reduction in the odds of good outcome compared with those with a baseline core volume less than 50 cm3 (those with volumes of 50-70 cm3: aOR, 0.29 [95% CI, 012-0.70]; P = .005; those with volumes >70 cm3: aOR, 0.30 [95% CI, 0.16-0.56]; P < .001).
Nonimaging Variables Independently Correlating With Good Outcomes After EVT
Younger age (aOR, 1.13 [95% CI, 1.00-1.28]; P = .049 per year), milder stroke severity on the NIHSS (aOR, 0.71 [95% CI, 0.54-0.93]; P = .01 with each NIHSS score increment), and shorter times from stroke onset to EVT (aOR, 0.60 [95% CI, 0.36-0.99]; P = .045 per hour) were independently correlated with good outcome (eTable 5 in the Supplement). There was a very low (<10%) probability of achieving functional independence when EVT occurred more than 12 hours after the time that the patient was last known to be well (Figure 3D).
Discussion
Patients with an acute ischemic stroke with large-vessel occlusion presenting with a substantial area of infarcted tissue visible on imaging is an important subpopulation. They made up almost one-fourth of all patients enrolled in the SELECT study and thus this cohort. Treating physicians face uncertainty on whether to proceed with EVT in these patients because of the lack of evidence from randomized clinical trials supporting the efficacy of the intervention and safety concerns regarding the consequences of futile reperfusion of large territories of established ischemia.
Large cores on CTP imaging was assessed in a pooled analysis of 50 patients with ischemic core volume of 70 cm3 or greater from the 7 clinical trials in the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration. Endovascular thrombectomy was associated with significant improvement in 90-day mRS score distribution (functional independence rate in an unadjusted analysis: EVT group, 2 of 25 [8%] vs medical management, 0 of 25 [0%]; common OR, 3.1 [95% CI, 1.0-9.4]).10 The treatment outcome, however, disappeared after adjusting for age and NIHSS scores.
In this cohort, we compared outcomes for patients who received EVT vs those who received medical management only. These nonrandomized data suggest better rates of functional independence with EVT and a shift toward better overall 90-day mRS scores. These benefits, however, did not reach significance after adjusting for baseline imbalances. The small sample size limits the power of this analysis.
In regards to safety, the rates of symptomatic ICH were similar between the treatment groups, and the mortality rate was lower in patients treated with EVT. Also, EVT was associated with smaller final infarcts and less infarct growth from presentation than medical management. These findings may represent surrogates for potential clinical benefit in this population with large strokes.
Successful reperfusion was associated with higher functional independence rates in patients treated with EVT in this cohort, but this finding also did not reach significance. Younger patients and those with milder stroke severity at presentation were more likely to achieve functional independence after EVT, which is consistent with previous reports in the literature.11
Previous reports indicated that good clinical outcomes with EVT decrease as ischemic core size increases.12 A post hoc analysis of the HERMES meta-analysis data10 showed that the increase in ischemic core volume is associated with decrease in good outcomes, albeit with potential benefit in patients up to 125 cm3. However, those trials largely excluded patients with substantial ischemic core volumes, and the number of patients with large strokes was very limited (to 50 patients with volumes >70 cm3).
We have assessed the outcome of different volume thresholds (50 cm3, 70 cm3, and 100 cm3) on the 90-day mRS distribution. The likelihood of good outcome with EVT declined as the volume increased, with a decline of 42% likelihood of good outcome for increase of every 10 cm3 of ischemic core volume. These data suggest reasonable rates of functional independence in patients with ischemic core volumes of 50 to 100 cm3. In 10 patients with volumes greater than 100 cm3, no functional independence was observed with EVT. Furthermore, symptomatic hemorrhage, neurological worsening, and mortality rates increased with the ischemic core size increment and was significantly higher in those with a volume greater than 100 cm3. There was, however, a limited number of patients in this group (10 patients treated with EVT and 15 patients treated with medical management only). Also, the rates of good outcomes in patients with very large ischemic core lesions also deteriorated in those who received medical management only. Thus, while treatment outcome determination can only be achieved in a randomized study, it is possible that the treatment outcome for a shift toward better outcomes may be still maintained in patients with larger core volumes.
Stroke size can be estimated on both CT and perfusion imaging. The 2 modalities measure different radiographic and physiological parameters. Computed tomography measures tissue hypodensities, whereas perfusion imaging measures the cerebral blood flow and volume. Computed tomography is a fast, inexpensive, and a universally readily available modality. However, it has a few limitations, such as the low interrater reliability13,14 and inability to provide ischemic core volume information or mismatch data. While perfusion imaging with an automated software can provide precise ischemic core volumes and mismatch data, this comes with a higher cost and infrastructure requirements and entails the use of contrast dye. It may also be associated with technical difficulties, such as motion artifacts and inappropriate bolus timing.
This study has estimated the early ischemic core size on both CT and CTP, and the data suggest the potential for better outcomes in patients treated with EVT compared with medical management alone using either imaging modality. While both CT and CTP can identify those who may achieve functional independence with EVT, these data suggest that CTP may be able to better identify patients with very large ischemic core volumes who have high mortality and symptomatic ICH rates after EVT. A decrease in ASPECTS on CT did not show a clear association with safety and poor functional outcomes; thus, the association between outcomes and ASPECTS was not as linear as ischemic core volume per CTP was. This could be associated with the low number of patients in each ASPECTS category. The differences in the outcomes associated with ischemic cores as measured by CTP and ASPECTS on functional outcomes can also indicate differences between 2 modalities in their ability to detect the volume of early tissue injury.
These data do not provide a definitive answer as to which modality should be used for identifying patients with large cores who would benefit from EVT. Data from randomized clinical trials are required to answer this question, and these are upcoming. These data provide hypothesis-generating evidence that patients with large cores visible on CT or CTP may achieve a reasonable rate of functional independence after EVT. Since CT is the standard imaging modality for assessing patients with strokes, treating physicians must use their judgment when interpreting evidence of large infarction on CT if CTP imaging is not available to quantify the volume of the early ischemic cores.
Unlike patients with small ischemic core volume, for whom the robustness of EVT benefit has been proven regardless of time in both DAWN and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) trials, it appears that the influence of time is more pronounced with increased ischemic core volume. This analysis showed the likelihood of good outcome with EVT declined for each hour of treatment delay, with a very low likelihood of benefit beyond 12 hours from the time that the patient was last seen well to treatment. These findings contrast with that of patients with small cores, in whom robust treatment outcomes have been documented up to 24 hours after onset.6,7 This may be attributable to the fact that patients with small cores had slow progression, which allowed for a later treatment benefit. In contrast, patients with large cores already have substantial brain injury; thus, the outcomes associated with time on EVT outcomes will be more pronounced. These data showed that the association between a large core as defined on different imaging modalities (CT and CTP) and EVT outcomes is affected by time.
In regards to CTP, the ischemic core volume and time to treatment both seem to affect the probability of functional independence. While late-window randomized clinical trials demonstrated EVT safety and efficacy to be stable over time in patients with small core volumes, this may not be the case in patients with large-core infarcts. In patients with large cores on CT, these data showed that lower ASPECTS on CT do not affect the probability of functional independence with EVT as much as the time delay does. This, however, could be attributable to the limited number of patients in each ASPECTS category in this cohort. Still, though, it is very difficult to form strong conclusions regarding these complex clinical associations based on these nonrandomized data with a limited sample size. Whether core size selection for EVT should vary with time or there is a time-invariant threshold remains to be seen. Prospective randomized data in a larger cohort of patients with large cores from a randomized clinical trial may help clarify these associations.
This study has several strengths. The SELECT study was a large prospective, multicenter cohort study with patients receiving unified imaging profile with a blinded independent core laboratory and blinded outcome assessors. This patient population was enrolled up to 24 hours after onset, which allowed for assessment of patients with large cores in both the early and late windows. The availability of both CT and CTP images in all patients allowed for evaluation of large core outcomes on both imaging modalities.
Currently, randomized clinical trials (SELECT2: A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke, Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window [TENSION],15 and In Extremis) are in the planning stages to evaluate the efficacy and safety of EVT in these patients, most of them using only ASPECTS on CT or MRI to classify patients with large cores. These results show that the treatment outcome of EVT appears to be negatively influenced by the core size with significant safety concerns in patients with very large cores (>100 cm3). Thus, a study that includes both CT and perfusion imaging will be able to assess not only EVT efficacy but also if both imaging modalities can identify patients who may be treated safely with EVT and achieve better outcomes than medical management. Moreover, it will allow for an assessment of the infarct volume on EVT efficacy and safety. We have incorporated this to the design of SELECT 2, a randomized clinical trial that is evaluating EVT efficacy and safety in patients with large cores with an adaptive design to address the association of volume and time with EVT outcomes.
Limitations
Although this work provides suggestive evidence that EVT may be efficacious in patients with large cores, it has many limitations. While SELECT was a prospective study, the retrospective nature of the analyses carries inherent limitations. Another major limitation is the lack of randomization into EVT vs medical management, which likely resulted in selection bias. In particular, the patients in the medical management group presented for evaluation later than the patients who received EVT and had larger ischemic core lesions at baseline. As a result, the models were adjusted for presentation time, core volume, and other factors associated with outcomes. Patients with more substantial medical comorbidities were probably less likely to be treated with EVT, and this factor is challenging to adjust for. While this cohort is one of the largest reported for large-core studies, the number of patients treated with ischemic core size of greater than 100 cm3 was small. We also had relatively few patients with large cores who were treated in the late window, which may have been associated with the fact that the DEFUSE 37and DAWN6 trials excluded patients with large cores. Also, only 1 patient with no mismatch on CTP imaging was enrolled. As the no-mismatch profile is not uncommon in patients treated in the late window, this suggests that the investigators were less likely to enroll these patients in the SELECT trial. Also, none of the patients with ASPECTS of 0 to 2 were treated with EVT. This could highlight the current practice of treating physicians excluding these patients because of the presumed low likelihood of functional independence with considerable safety concerns. However, none of the patients with ASPECTS of 0 to 2 who were treated with medical management only achieved functional independence, either. Furthermore, restricting the analysis to patients with ASPECTS of 3 to 5, functional independence rates were still higher in patients treated with EVT as well. Whether EVT may result in better functional outcomes without increasing adverse safety events in patients with ASPECTS of 0 to 2 remains to be seen.
These data analyses assessed stroke size on CT and CTP, but we did not have an MRI assessment prior to the thrombectomy procedure. Some centers use MRI to triage patients prior to thrombectomy procedures. Prior studies have suggested better functional outcomes with successful reperfusion in patients with large cores, as measured by diffusion-weighted images changes.16,17,18,19 Whether EVT is safe and efficacious in patients with large cores on MRI remains to be seen. A future randomized clinical trial, the In Extremis study, will assess the EVT outcomes in patients with large cores on MRI.
Conclusions
In patients with large cores on CT or CTP images, EVT resulted in reasonable rates of functional independence with acceptable safety. The potential benefit of EVT is likely less and the risks of hemorrhage greater in patients who present late or have very large core sizes (>100 mL). Future clinical trials aimed at addressing the efficacy and safety of EVT in patients with large cores are warranted and under way.
eAppendix. Study personnel.
eTable 1. Univariable and multivariable logistic regression models comparing functional independence (90 day mRS 0-2) in patients treated with thrombectomy versus medical management only.
eTable 2. Univariable and multivariable ordinal logistic regression models comparing 90 day modified Rankin Scale score distribution (shift) in patients treated with thrombectomy versus medical management only.
eTable 3. Univariable and multivariable logistic and ordinal logistic regression models comparing functional independence (90 day mRS 0-2) in patients treated with thrombectomy versus medical management only in patients with large core on different imaging modalities.
eTable 4. Rates of functional independence in patients receiving thrombectomy and medical management only, stratified by ASPECTS score. ASPECTS: Alberta Stroke Program Early CT Score.
eTable 5. Multivariable logistic regression model identifying variables independently associated with functional independence (90 day mRS 0-2) in patients treated with endovascular thrombectomy.
eTable 6. Clinical and Radiographic Outcomes of the cohort divided based on the treatment received in patients with ASPECTS ≤ 6.
eFigure 1. Depicts 90 day modified Rankin Scale score distribution in large core patients treated with endovascular thrombectomy as compared to those who were treated by medical management only by imaging modality a) Large core on CT, b) Large core on CTP, c) Large core on CT & CTP.
eFigure 2. Depicts 90 day modified Rankin Scale score distribution in patients who achieved successful reperfusion (mTICI ≥ 2b) with endovascular thrombectomy as compared to those who did not (mTICI=0-2a).
eFigure 3. Illustrates 90 day modified Rankin Scale score distribution stratified by ischemic core volume on CT perfusion (50-100 cc) vs. > 100 cc.
eFigure 4. The probability of good outcomes at 90 day in patients who were treated with endovascular thrombectomy in relation to ASPECTS score.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 Hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/str.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 10.Campbell B, Majoie C, Albers G, et al. ; HERMES Collaborators . Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46-55. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 12.Xie Y, Oppenheim C, Guillemin F, et al. ; THRACE investigators . Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol. 2018;83(1):178-185. doi: 10.1002/ana.25133 [DOI] [PubMed] [Google Scholar]
- 13.Naylor J, Churilov L, Chen Z, Koome M, Rane N, Campbell BCV. Reliability, reproducibility and prognostic accuracy of the Alberta Stroke Program early CT score on CT perfusion and non-contrast CT in hyperacute stroke. Cerebrovasc Dis. 2017;44(3-4):195-202. doi: 10.1159/000479707 [DOI] [PubMed] [Google Scholar]
- 14.van Seeters T, Biessels GJ, Niesten JM, et al. ; Dust Investigators . Reliability of visual assessment of non-contrast CT, CT angiography source images and CT perfusion in patients with suspected ischemic stroke. PLoS One. 2013;8(10):e75615. doi: 10.1371/journal.pone.0075615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendszus M, Bonekamp S, Berge E, et al. . A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int J Stroke. 2019;14(1):87-93. doi: 10.1177/1747493018798558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panni P, Gory B, Xie Y, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators . Acute stroke with large ischemic core treated by thrombectomy. Stroke. 2019;50(5):1164-1171. doi: 10.1161/STROKEAHA.118.024295 [DOI] [PubMed] [Google Scholar]
- 17.Gilgen MD, Klimek D, Liesirova KT, et al. . Younger stroke patients with large pretreatment diffusion-weighted imaging lesions may benefit from endovascular treatment. Stroke. 2015;46(9):2510-2516. doi: 10.1161/STROKEAHA.115.010250 [DOI] [PubMed] [Google Scholar]
- 18.Desilles JP, Consoli A, Redjem H, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Research Investigators . Successful reperfusion with mechanical thrombectomy is associated with reduced disability and mortality in patients with pretreatment diffusion-weighted imaging-Alberta Stroke Program early computed tomography score ≤6. Stroke. 2017;48(4):963-969. doi: 10.1161/STROKEAHA.116.015202 [DOI] [PubMed] [Google Scholar]
- 19.Manceau PF, Soize S, Gawlitza M, et al. . Is there a benefit of mechanical thrombectomy in patients with large stroke (DWI-ASPECTS ≤ 5)? Eur J Neurol. 2018;25(1):105-110. doi: 10.1111/ene.13460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Study personnel.
eTable 1. Univariable and multivariable logistic regression models comparing functional independence (90 day mRS 0-2) in patients treated with thrombectomy versus medical management only.
eTable 2. Univariable and multivariable ordinal logistic regression models comparing 90 day modified Rankin Scale score distribution (shift) in patients treated with thrombectomy versus medical management only.
eTable 3. Univariable and multivariable logistic and ordinal logistic regression models comparing functional independence (90 day mRS 0-2) in patients treated with thrombectomy versus medical management only in patients with large core on different imaging modalities.
eTable 4. Rates of functional independence in patients receiving thrombectomy and medical management only, stratified by ASPECTS score. ASPECTS: Alberta Stroke Program Early CT Score.
eTable 5. Multivariable logistic regression model identifying variables independently associated with functional independence (90 day mRS 0-2) in patients treated with endovascular thrombectomy.
eTable 6. Clinical and Radiographic Outcomes of the cohort divided based on the treatment received in patients with ASPECTS ≤ 6.
eFigure 1. Depicts 90 day modified Rankin Scale score distribution in large core patients treated with endovascular thrombectomy as compared to those who were treated by medical management only by imaging modality a) Large core on CT, b) Large core on CTP, c) Large core on CT & CTP.
eFigure 2. Depicts 90 day modified Rankin Scale score distribution in patients who achieved successful reperfusion (mTICI ≥ 2b) with endovascular thrombectomy as compared to those who did not (mTICI=0-2a).
eFigure 3. Illustrates 90 day modified Rankin Scale score distribution stratified by ischemic core volume on CT perfusion (50-100 cc) vs. > 100 cc.
eFigure 4. The probability of good outcomes at 90 day in patients who were treated with endovascular thrombectomy in relation to ASPECTS score.