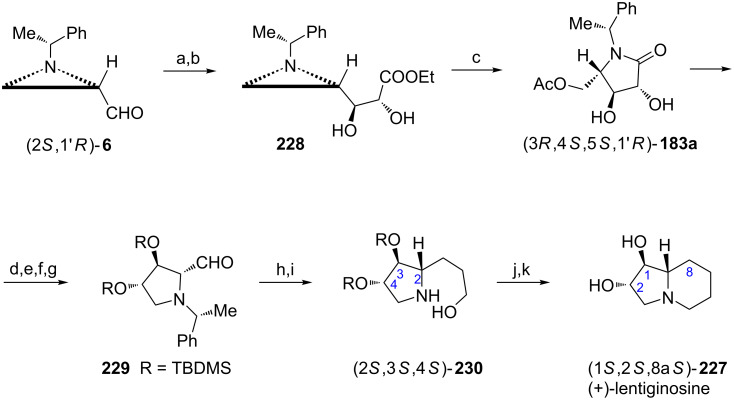
Scheme 57.
Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, LiHMDS, THF, rt, 1 h; b) AD-mix-α, MeSO2NH2, t-BuOH/H2O, 0 °C, 24 h; c) AcOH, CH2Cl2, rt, 12 h, then toluene, 90 °C, 12 h; d) TfOTBDMS, 2,6-lutidine, CH2Cl2, 0 °C, 1 h; e) KOH, EtOH, rt, 1 h; f) BH3·SMe2, THF, 0 °C to 70 °C, 3 h; g) Swern oxidation; h) Ph3P+(CH2)3OBn Brˉ, t-BuOK, THF, 0 °C to rt, 1 h; i) H2, Pd(OH)2, CF3COOH, MeOH, rt, 24 h; j) CBr4, Ph3P, TEA, CH2Cl2, 0 °C to rt, 6 h; k) HCl, MeCN, rt, 4 h.