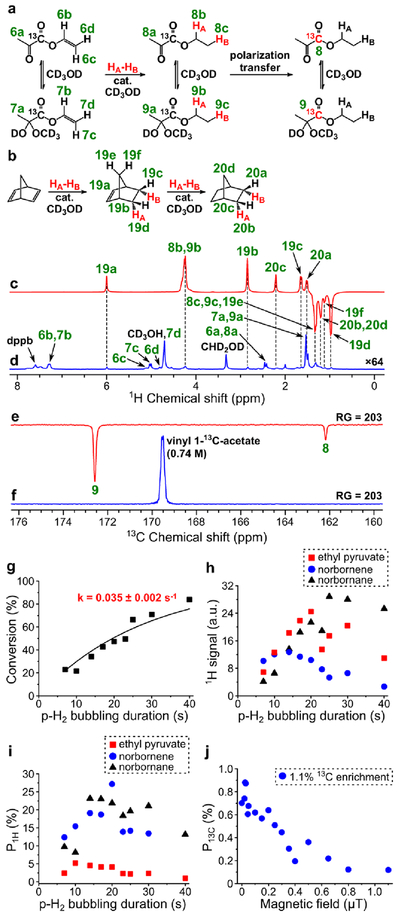
Figure 5.
(a) Reaction scheme of pairwise addition of p-H2 to vinyl 1-13C-pyruvate in CD3OD followed by polarization transfer to 13C nuclei (HA and HB are two atoms from the same p-H2 molecule, cat. = [Rh(NBD)(dppb)]BF4). (b) Reaction scheme of the competing process of norbornadiene hydrogenation with p-H2. (c) 1H NMR spectrum acquired after 1H ALTADENA hyperpolarization of ethyl 1-13C-pyruvate with 10 s p-H2 bubbling duration. (d) Corresponding thermal 1H NMR spectrum acquired after relaxation of hyperpolarization (multiplied by a factor of 64). ε1H = 1650, P1H = 5.1% (5.2% at 85% p-H2 fraction) (calculated using signal 8b+9b). (e) 13C NMR spectrum acquired after 13C hyperpolarization of ethyl pyruvate (1.1% 13C enrichment) using MFC at 0.025 μT magnetic field with RG = 203 (p-H2 bubbling duration = 20 s). (f) 13C NMR spectrum of 0.74 M solution of vinyl 1-13C-acetate used as an external reference acquired with RG = 203. ε13C = 900, P13C = 0.70% (0.88% at 85% p-H2 fraction). (g) Dependence of conversion of vinyl pyruvate to ethyl pyruvate on p-H2 bubbling duration (estimated pseudo-first order rate constant k = 0.035 ± 0.002 s–1). (h) Dependence of 1H ALTADENA signal (absolute value) of HP ethyl pyruvate (signal 8b+9b, red squares), norbornene (signal 19d, blue circles) and norbornane (signal 20b+20d, black triangles) on p-H2 bubbling duration. (i) Dependence of P1H (at 85% p-H2 fraction) of ethyl pyruvate (signal 8b+9b, red squares), norbornene (signal 19d, blue circles) and norbornane (signal 20b+20d, black triangles) on p-H2 bubbling duration. (j) Dependence of P13C (at 85% p-H2 fraction) of ethyl pyruvate on magnetic field used in MFC experiments (data obtained with the 1.1% 13C-enriched precursor).