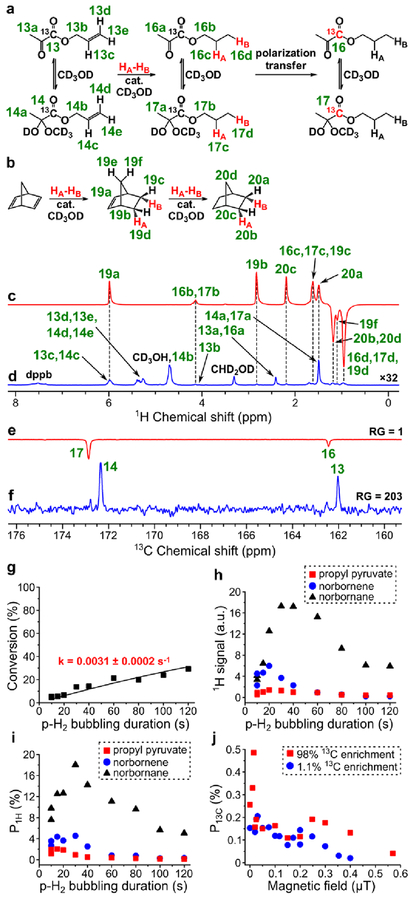
Figure 6.
(a) Reaction scheme of pairwise addition of p-H2 to allyl 1-13C-pyruvate in CD3OD followed by polarization transfer to 13C nuclei (HA and HB are two atoms from the same p-H2 molecule, cat. = [Rh(NBD)(dppb)]BF4). (b) Reaction scheme of the competing process of norbornadiene hydrogenation with p-H2. (c) 1H NMR spectrum acquired after 1H ALTADENA hyperpolarization of propyl 1-13C-pyruvate with 15 s p-H2 bubbling duration. (d) Corresponding thermal 1H NMR spectrum acquired after relaxation of hyperpolarization (multiplied by a factor of 32). ε1H = 480, P1H = 1.5% (2.0% at 85% p-H2 fraction) (calculated using signal 16b+17b). (e) 13C NMR spectrum acquired after 13C hyperpolarization of propyl 1-13C-pyruvate using MFC at 0.015 μT magnetic field with RG = 1. (f) Corresponding thermal 13C NMR spectrum acquired after relaxation of hyperpolarization with RG = 203. ε13C = 610, P13C = 0.47% (0.49% at 85% p-H2 fraction). (g) Dependence of conversion of allyl pyruvate to propyl pyruvate on p-H2 bubbling duration (estimated pseudo-first order rate constant k = 0.0031 ± 0.0002 s–1). (h) Dependence of 1H ALTADENA signal (absolute value) of HP propyl pyruvate (signal 16b+17b, red squares), norbornene (signal 19b, blue circles) and norbornane (signal 20b+20d, black triangles) on p-H2 bubbling duration. (i) Dependence of P1H (at 85% p-H2 fraction) of allyl acetate (signal 16b+17b, red squares), norbornene (signal 19b, blue circles) and norbornane (signal 20b+20d, black triangles) on p-H2 bubbling duration. (j) Dependence of P13C (at 85% p-H2 fraction) of propyl 1-13C-pyruvate on magnetic field used in MFC experiments (red squares – data points obtained with the 98% 13C-enriched precursor, blue circles – data points obtained with the 1.1% 13C-enriched precursor).