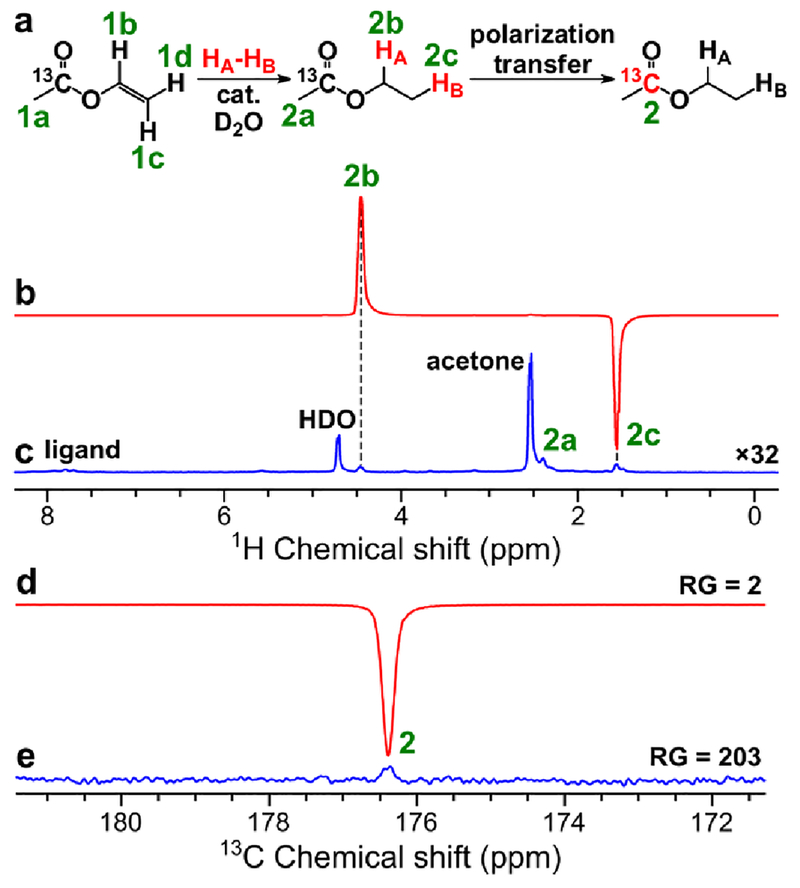
Figure 8.
(a) Reaction scheme of pairwise addition of p-H2 to vinyl 1-13C-acetate in D2O over water-soluble Rh catalyst followed by polarization transfer to 13C nuclei (HA and HB are two atoms from the same p-H2 molecule). (b) 1H NMR spectrum acquired after 1H ALTADENA hyperpolarization of ethyl 1-13C-acetate with 7 s p-H2 bubbling duration at 70 psig and 60 °C. (c) Corresponding thermal 1H NMR spectrum acquired after relaxation of hyperpolarization (multiplied by a factor of 32). Acetone was used during sample preparation step.62 ε1H = 1680, P1H = 5.2% (5.3% at 85% p-H2 fraction) (calculated using signal 2b). (d) 13C NMR spectrum acquired after 13C hyperpolarization of ethyl 1-13C-acetate using MFC at near 0 μT magnetic field with RG = 2 (p-H2 bubbling duration = 7 s at 70 psig and 60 °C). (e) Corresponding thermal 13C NMR spectrum acquired after relaxation of hyperpolarization with RG = 203. ε13C = 1550, P13C = 1.2% (2.1% at 85% p-H2 fraction).