Table 1.
Selected electrophilic activators of NRF2 under clinical development.
| Compound | Type | Mechanism of action | Disease | Clinical trial | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
Bardoxolone-methyl (CDDO-Me)
|
Synthetic triterpenoids | Electrophilic modification of KEAP1-Cys-151 | Diabetic nephropathy | Phase II | NCT00811889 |
| IgA nephropathy CKD associated with type 1 diabetes Focal segmental glomerulosclerosis Autosomal dominant polycystic kidney |
Phase II | NCT03366337 | |||
| Chronic kidney disease Type 2 diabetes Diabetic nephropathy |
Phase III | NCT01351675 | |||
| Liver disease | Phase I/II | NCT00550849 | |||
| Hepatic impairment Healthy |
Phase I | NCT01563562 | |||
| Advanced solid tumors lymphoid malignancies | Phase I |
NCT00529438
NCT00508807 |
|||
| Alport syndrome | Phase II/III cardinal | NCT03019185 | |||
| Pulmonary hypertension | Phase III RANGER | NCT03068130 | |||
| Pulmonary arterial hypertension | Phase III | NCT02657356 | |||
| Renal insufficiency, chronic Diabetes mellitus, type 2 |
Phase II | NCT01053936 | |||
|
| |||||
RTA-408 (omaveloxolone)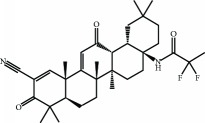
|
Synthetic triterpenoids | Electrophilic modification of KEAP1-Cys-151 | Mitochondrial myopathy | Phase II | NCT02255422 |
| Friedreich's ataxia | Phase II | NCT02255435 | |||
| Inflammation and pain following ocular surgery | Phase II | NCT02065375 | |||
| Corneal endothelial cell loss Ocular pain Ocular inflammation Cataract surgery |
Phase II | NCT02128113 | |||
| Melanoma | Phase I/II | NCT02259231 | |||
| Breast cancer | Phase II | NCT02142959 | |||
|
| |||||
Dimethyl fumarate
|
Fumaric acid ester | Electrophilic modification of KEAP1-Cys-151 | Multiple sclerosis | Approved | |
| Psoriasis | Approved | ||||
| Rheumatoid arthritis | Phase II | NCT00810836 | |||
| Adult brain glioblastoma | Phase I | NCT02337426 | |||
| Cutaneous T cell lymphoma | Phase II | NCT02546440 | |||
| Obstructive sleep apnea | Phase II | NCT02438137 | |||
| Chronic lymphocytic leukemia Small lymphocytic lymphoma |
Phase I | NCT02784834 | |||
|
| |||||
ALKS-8700
|
Fumaric acid ester (MMF-derivate) | Electrophilic modification of KEAP1-Cys-151 | Multiple sclerosis | Phase III | NCT02634307 |
|
| |||||
Oltipraz
|
Organosulfur compound | Electrophilic modification of KEAP1-Cys-151 | Nonalcoholic steatohepatitis | Phase III | NCT02068339 |
| Schistosomiasis | Approved | ||||
| Lung cancer | Phase I | NCT00006457 | |||
|
| |||||
Ursodiol
|
Biliary acid | Electrophilic modification of KEAP1-Cys-151 | Cholestasis | Phase II/III | NCT00846963 |
| Diarrhea | Phase IV | NCT02748616 | |||
| Cholelithiasis | Phase III | NCT02721862 | |||
| Primary biliary cirrhosis | Phase IV | NCT01510860 | |||
| Barrett esophagus Low-grade dysplasia |
Phase II | NCT01097304 | |||
| Chronic hepatitis C | Phase III | NCT00200343 | |||
| Type 2 diabetes mellitus | Phase II | NCT02033876 | |||
|
| |||||
Sulforaphane
|
Isothiocyanate | Electrophilic modification of KEAP1-Cys-151 | Schizophrenia | Phase II/III | NCT02880462 |
| Phase II | NCT02810964 | ||||
| Phase II | NCT01716858 | ||||
| COPD | Phase II | NCT01335971 | |||
| Atopic asthmatics | Phase I | NCT01845493 | |||
| Autism spectrum disorder | Phase II | NCT01474993 | |||
| Phase II | NCT02909959 | ||||
| Phase II | NCT02677051 | ||||
| Phase II | NCT02654743 | ||||
| Phase I/II | NCT02561481 | ||||
| Healthy | Phase I | NCT01008826 | |||
| Phase I | NCT02023931 | ||||
| Melanoma | Phase I | NCT01568996 | |||
| Asthma | Phase I | NCT01845493 | |||
| Phase I/II | NCT01183923 | ||||
| Prostate cancer | Phase II | NCT01228084 | |||
| Breast cancer | Phase II | NCT00843167 | |||
| Lung cancer | Phase II | NCT03232138 | |||
| Environmental carcinogenesis | Phase II | NCT01437501 | |||
| Alcohol sensitivity | Phase II | NCT01845220 | |||
| Aging | Phase II | NCT03126539 | |||
| Rhinitis, allergic | Phase II | NCT02885025 | |||
| Helicobacter pylori infection | Phase IV | NCT03220542 | |||
| Diabetes mellitus, noninsulin-dependent | Phase II | NCT02801448 | |||
|
| |||||
Sulforadex (SFX-01)
|
Sulforaphane/alpha-cyclodextrin complex | Electrophilic modification of KEAP1-Cys-151 | Subarachnoid haemorrhage | Phase II | NCT02614742 |
| Breast neoplasm | Phase I/II | NCT02970682 | |||
| Prostate cancer | Phase I |
NCT02055716
NCT01948362 |
|||
|
| |||||
ITH12674
|
Melatonin-sulforaphane hybrid | Electrophilic modification of KEAP1-Cys-151 | Brain ischemia | Preclinical PK | No clinical trials available |
|
| |||||
Curcumin
|
Stilbene | Electrophilic modification of KEAP1-Cys-151 | Type 2 diabetes Prediabetes Insulin resistance Cardiovascular risk |
Phase IV | NCT01052025 |
| Schizophrenia Cognition Psychosis |
Phase I/II | NCT02104752 | |||
| Acute kidney injury Abdominal aortic aneurysm |
Phase II/III | NCT01225094 | |||
| Chronic kidney diseases Diabetes mellitus, type 2 Polymorphism |
Phase II/III | NCT03262363 | |||
| Alzheimer's disease | Phase I/II | NCT00164749 | |||
| Neoplasms | Phase II | NCT02944578 | |||
| Crohn's disease | Phase III | NCT02255370 | |||
| Chronic schizophrenia | Phase IV | NCT02298985 | |||
| Mild cognitive impairment | Phase II | NCT01811381 | |||
| Prostate cancer | Phase III | NCT02064673 | |||
| Major depression | Phase IV | NCT01750359 | |||
|
| |||||
Resveratrol
|
(E)-Stilbene derivate | Electrophilic modification of KEAP1-Cys-151 | Type 2 diabetes | Phase I | NCT01677611 |
| Colon cancer | Phase I | NCT00256334 | |||
| COPD | N/A | NCT02245932 | |||
| Friedreich ataxia | Phase I/II | NCT01339884 | |||
| Nonalcoholic fatty liver | Phase II/III | NCT02030977 | |||
| Nonischemic cardiomyopathy | Phase III | NCT01914081 | |||
| Endometriosis | Phase IV | NCT02475564 | |||
| Chronic renal insufficiency | Phase III | NCT02433925 | |||
| Metabolic syndrome X | Phase II | NCT02114892 | |||
| Chronic subclinical inflammation Redox status |
Phase III | NCT01492114 | |||
| Alzheimer's disease | Phase II | NCT01504854 | |||
| Phase III | NCT00743743 | ||||
| Huntington disease | Phase III | NCT02336633 | |||
|
| |||||
CXA-10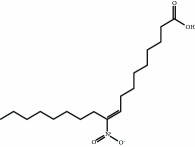
|
Nitro-fatty acid (NFA) | Electrophilic modification of KEAP1-Cys-273 and Cys-288 | Acute kidney injury | Phase I | NCT02248051 |
| Pulmonary arterial hypertension (PAH) | Phase II | NCT03449524 | |||
| Primary focal segmental glomerulosclerosis (FSGS) | Phase II | NCT03422510 | |||
