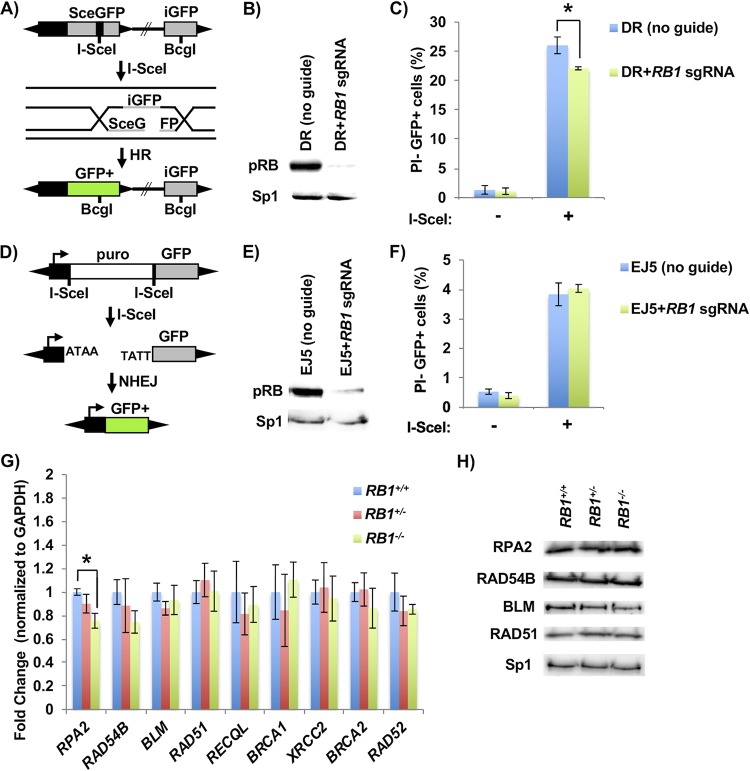
FIG 4.
Defective homology-directed repair of DNA breaks in RB1 mutant cells. (A) Schematic of the DR-GFP homology-directed repair construct used. Cleavage of an I-SceI site integrated into an expressed, but mutant, GFP gene (SceGFP) can be repaired from a downstream internal GFP fragment (iGFP). (B) U2OS cells with clonal integration of the HR reporter construct were ablated for RB1 expression with lentiviral delivery of Cas9 and an RB1-specific sgRNA. The relative expression of RB in the population of cells was determined by Western blotting, and Sp1 serves as a loading control. (C) HR repair efficiency of RB1 mutant U2OS cells was determined by transfecting an expression vector for I-SceI endonuclease (+), or a relevant negative-control expression vector, and quantitating PI-negative (PI−) and GFP-positive (GFP+) cells by flow cytometry (n = 5). All error bars are ±1 SEM. *, P < 0.05. (D) Schematic of the EJ5 NHEJ reporter system. DNA breaks at tandem I-SceI sites release the puromycin resistance gene, allowing NHEJ repair to join a promoter to the GFP-expressing sequence. (E) After the generation of a stable U2OS clone containing the NHEJ reporter construct, RB1 was deleted as described above and confirmed by Western blotting. (F) NHEJ repair efficiency was determined by transfecting an I-SceI endonuclease expression vector (+), or the negative control, and PI-negative and GFP-positive cells were quantitated by flow cytometry (n = 3). (G) RT-qPCR was performed to assess the transcript levels of various HR factors in RB1 wild-type (4 different clones), heterozygous (3 different clones), and knockout (4 different clones) cells. Statistical differences in means were determined by one-way ANOVA. All error bars are ±1 SEM. *, P < 0.05. (H) Western blots showing expression of various HR factors in wild-type, heterozygous, and homozygous RB1 mutant clones. The Sp1 loading control is shown on the bottom.