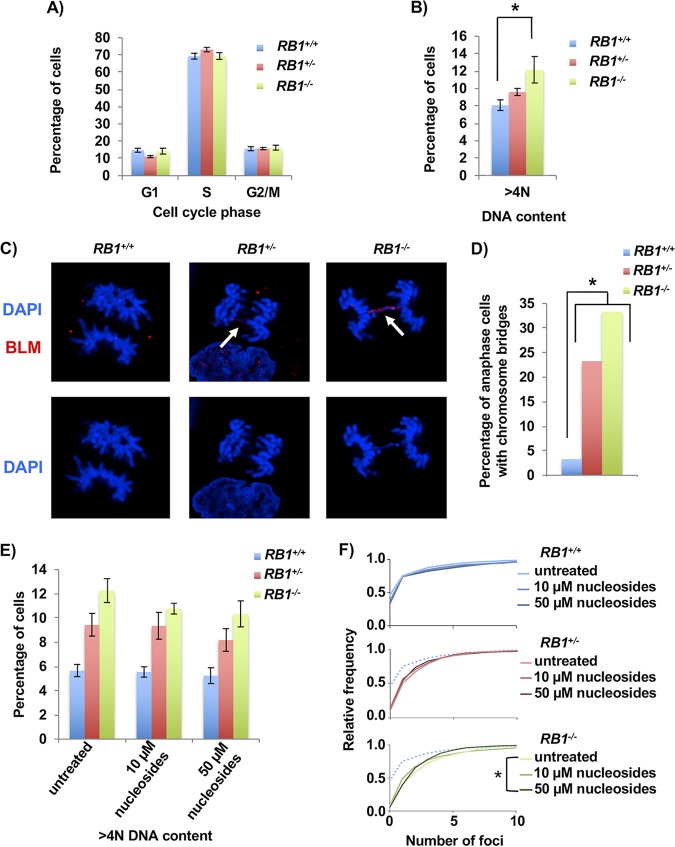
FIG 6.
Increased mitotic errors in RB1 mutant cells. (A) BrdU and propidium iodide staining followed by flow cytometry was used to determine the cell cycle phase distribution of asynchronous cultures of U2OS cells. Four clones for the wild-type and knockout genotypes and three clones for the heterozygous genotype were analyzed. (B) Flow cytometry analysis showing the proportion of cells with >4N DNA content. Means were compared using one-way ANOVA. (C) Cells in anaphase were imaged by fluorescence microscopy using DAPI (blue) and BLM (red) in cells from each RB1 genotype. Arrows indicate anaphase bridges that are stained by both DAPI and BLM. (D) Quantitation of the numbers of anaphase cells with DAPI-stained chromosome bridges. The proportion of cells with bridges is significantly higher in the knockout and heterozygous clones than in the wild-type clone, as determined by the χ2 test. (E) Representative clones from each RB1 genotype were either left untreated or treated with 10 μM or 50 μM nucleosides for 48 h. Flow cytometry analysis of propidium iodide-stained cells shows the proportion of cells with >4N DNA content. Mean differences were compared by one-way ANOVA (n = 3). (F) Nucleoside-treated cells were fixed and stained for γH2AX after 48 h of culture. DNA damage is summarized in frequency plots. The dotted line in the RB1+/− and RB1−/− cumulative frequency plots represents the wild-type untreated cells. Statistical significance between genotypes was determined by the Kolmogorov-Smirnov test. All error bars are ±1 SEM. *, P < 0.05.