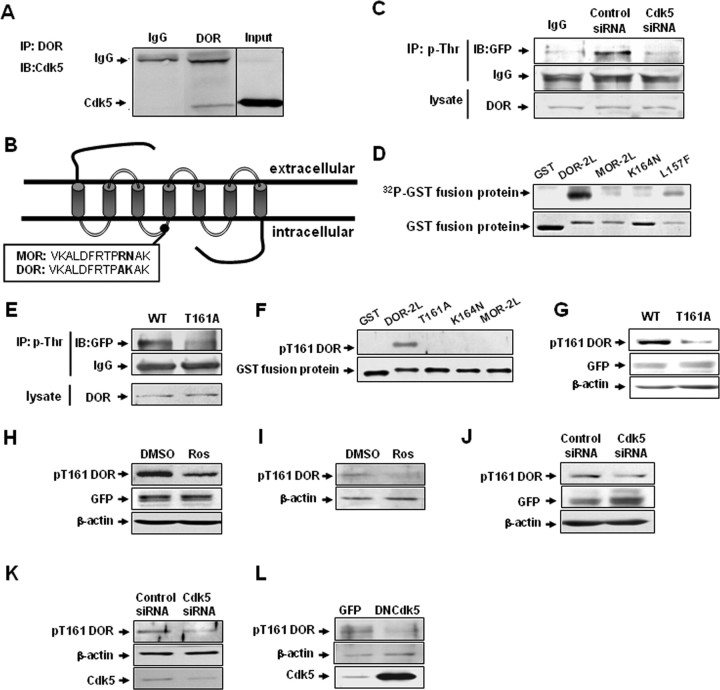
Figure 2.
Cdk5 phosphorylates DOR directly at Thr-161 in the second intracellular loop, and Lys-164 is necessary for this phosphorylation of Cdk5 in vitro and in vivo. A, Interaction of Cdk5 and DOR in rat DRGs. Cdk5-specific antibody recognized the complex immunoprecipitated (IP) by DOR-specific antibody, but normal IgG did not. B, Schematic representation of two opioid receptors, MOR and DOR, showing the putative Cdk5 phosphorylation site (TPAK) in the second intracellular loop in DOR, but not in MOR. C, Representative Western blots of DOR proteins after immunoprecipitation with a phosphothreonine-specific antibody in cells cotransfected with EGFP-DOR and control siRNA or Cdk5 siRNA. IgG was used as negative controls. Results are representative of three independent experiments. D, Cdk5 phosphorylates DOR at Thr-161. Purified GST and GST fusion proteins were used as substrates for the Cdk5 kinase assays. Cdk5 was immunoprecipitated from rat brain lysates using a Cdk5-specific antibody. Autoradiographs are shown at the top, and corresponding Coomassie Blue-stained gel are shown at the bottom. GST-DOR-2L and GST-DOR-2L-L157F were phosphorylated by Cdk5, whereas GST-DOR-2L-K164N and GST-MOR-2L were not. E, Representative Western blots of DOR proteins after immunoprecipitation with a phosphothreonine-specific antibody in cells transfected with wild-type DOR or the T161A mutant. F, In Cdk5 cold kinase assay, purified GST and GST fusion proteins were immunodetected with pT161 DOR phosphoantibody, showing specificity of the pT161 DOR phosphoantibody for the wild-type GST-DOR-2L. G, NG108-15 cells were transfected with wild-type DOR and DOR mutant T161A, and the blots were immunodetected with site-specific pT161 DOR antibody. Total cell lysates were immunoblotted with GFP and actin antibodies as a loading control. H, Cdk5 inhibitor roscovitine reduces DOR Thr-161 phosphorylation. NG108-15 cells were transfected with GFP-DOR and treated for 4 h with the indicated drugs, and then immunoblotted with pT161 DOR antibody. Total cell lysates were immunoblotted with GFP and actin antibodies as loading controls. I, Effect of roscovitine on Thr-161 phosphorylation of endogenous DOR in primary cultures of DRG neurons. Blots were reprobed with anti-actin antibody to ensure equal loading. J, Cdk5 siRNA reduces DOR Thr-161 phosphorylation. NG108-15 cells were cotransfected with GFP-DOR and control or specific Cdk5 siRNA, and then immunoblotted with pT161 DOR antibody. Total cell lysates were immunoblotted with GFP and actin antibodies as a loading control. K, Effect of Cdk5 siRNA on Thr-161 phosphorylation of endogenous DOR in primary cultures of DRG neurons. Blots were reprobed with anti-actin antibody to ensure equal loading. L, Effect of Cdk5 on Thr-161 phosphorylation of endogenous DOR in primary cultures of DRG neurons infected with adenovirus driving expression of GFP alone or GFP together with DNCdk5.