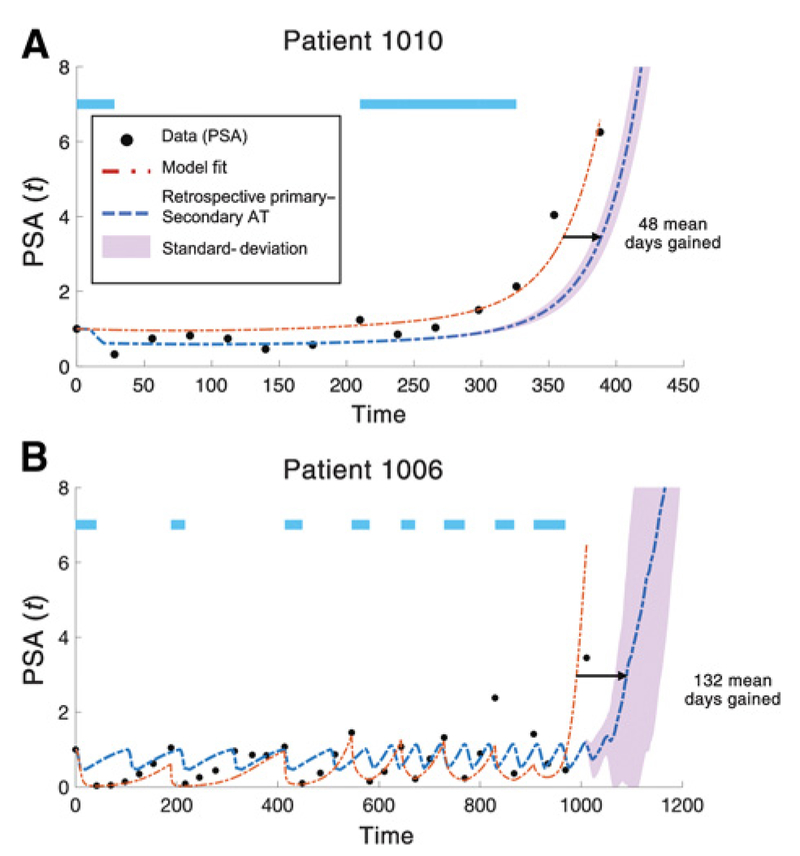
Figure 5.
Days gained via P-S therapy. Data from two of the four patients with PSA and radiographic progression. Model fit (dashed red line) shown for identical parameters as Fig. 3 except for initial conditions . The model accurately recapitulates time to relapse due to resistant T− population. Parameters from patient-specific model fitting are used to test the efficacy of the addition of secondary docetaxel treatment at the optimal time of each abiraterone cycle (blue dashed line; Δt = 30). We use a “days gained” metric to note the extension in days the model predicts a 3-fold (PSA = 3) increase in PSA from the initial value for abiraterone-docetaxel adaptive therapy compared with adaptive abiraterone only. A, Patient 1010 relapses after two cycles of abiraterone. Best model fit parameters: x1(0) = 1.4 × 102; x2(0) = 6.9 × 102;x3(0) = 9.2 × 10−3. Docetaxel as a secondary therapy predicts an average of 48 days gained (blue dashed line). B, Patient 1006 relapses after eight cycles of abiraterone. Best model fit parameters: x1(0) = 8.7 × 102; x2(0) = 1.6 × 10−5; x3(0) = 4.0 × 10−25. Docetaxel as a secondary therapy predicts an average of 132 days gained (blue dashed line). This “days gained” metric depends on the initial condition of T−/−, but the model predicts a positive value for days gained for a range of T−/− values. Purple-shaded region shows P-S deviation for a range of 5 orders of magnitude of the initial T−/− population such that x4(0) ∈ [x3(0)/105, x3(0)].