Figure 3.
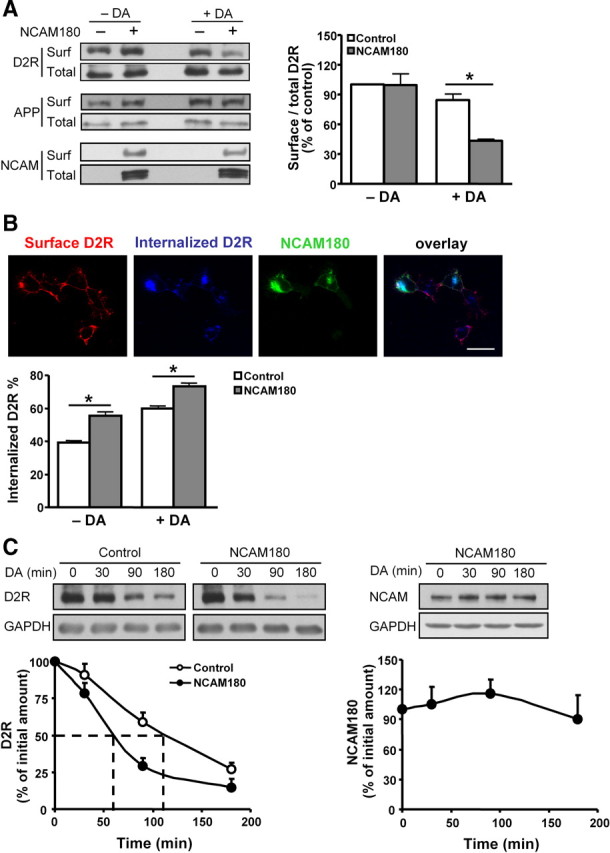
Regulation of D2R trafficking by NCAM. A, Myc-D2R-expressing HEK293 cells were mock-transfected or transfected with NCAM180. Two days later, cells were stimulated with 10 μm DA for 30 min, followed by cell surface biotinylation with Sulfo-NHS-LC-biotin. Biotin-labeled surface proteins (surf) and total proteins (total) were detected by Western blot analysis using antibodies against D2R, APP, or NCAM, respectively. The levels of selected proteins at the cell surface relative to their total levels were determined, with the ratio obtained from the mock-transfected cells in the absence of dopamine being set to 100%. *p < 0.05 by unpaired t test (n = 3). B, Myc-D2R-expressing HEK293 cells transfected with NCAM180 were subjected to live staining with myc antibody to label cell surface D2Rs. After treatment with 10 μm dopamine, cells were stained with antibodies against D2R before permeabilization (surface D2Rs) and after permeabilization (internalized D2Rs). Percentage of internalized D2Rs, defined as the ratio of internalized to total fluorescence intensities, was presented as mean + SEM for 50 cells. Results are representative of two independent experiments. *p < 0.05 by unpaired t test. Scale bar, 25 μm. C, Myc-D2R-expressing HEK293 cells transiently transfected with NCAM180 were stimulated with 10 μm DA for 0, 30, 90, or 180 min in the presence of 10 μg/ml cycloheximide to block de novo protein synthesis. The levels of D2R and NCAM were evaluated by Western blot analysis with D2R and NCAM antibodies, respectively. Determination of GAPDH levels served as control for loading. The proteins levels obtained by dopamine stimulation for 0 min were set to 100%. The dashed lines indicate 50% degradation of D2Rs. Means + SEM are shown here (n = 3).
