Figure 4.
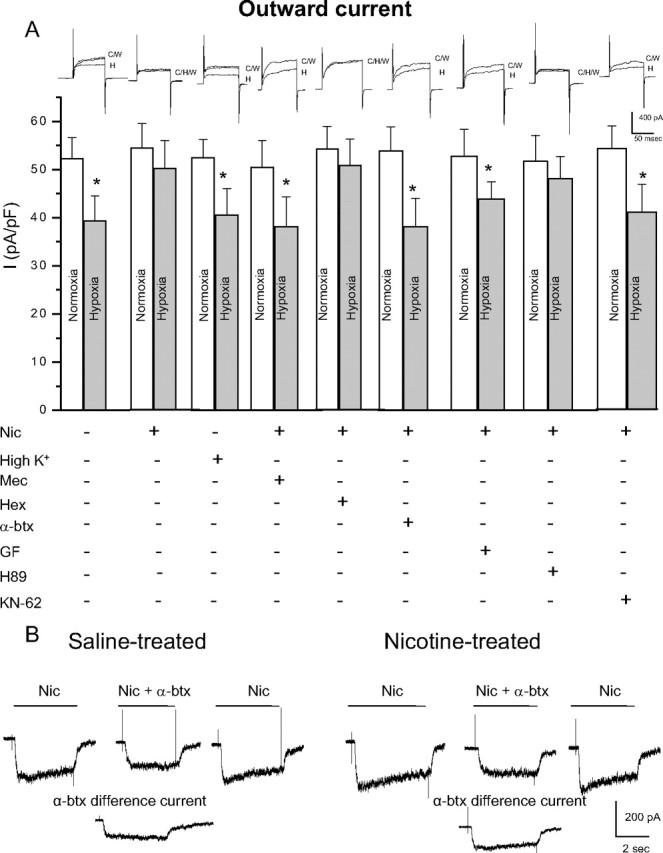
Role of nAChR subtypes and intracellular signaling pathways in the nicotine-mediated loss of hypoxic sensitivity in neonatal AMCs. Dissociated AMCs from saline-treated P0 pups were grown for ∼1 week in culture under control conditions, or in presence of nicotine base (Nic) (50 μm) with either mecamylamine (Mec) (100 μm; general nAChR blocker), hexamethonium (Hex) (100 μm; blocker of most nAChRs except α7), α-bungarotoxin (α-btx) (100 nm; specific α7 nAChR blocker), or the depolarizing stimulus high K+ (30 mm) as indicated. To probe the signaling pathways, neonatal AMCs were cultured with nicotine and one of the following: the PKC blocker GF109203X (GF) (2 μm), CaM kinase inhibitor KN-62 (3 μm), or the PKA inhibitor H-89 (20 μm). Histogram indicates (mean ± SEM) outward current density (in picoamperes per picofarad) at +30 mV during normoxia or hypoxia (A). Note that hypoxic sensitivity, indicated by inhibition of outward current, was lost after chronic nicotine treatment in culture and that this effect was prevented by α7 nAChR antagonists Mec and α-btx only. Also, hypoxia sensitivity was detectable in nicotine-treated AMCs after coincubation with the PKC inhibitor GF, the CaM kinase inhibitor KN-62, but not the PKA inhibitor H-89. Representative traces for each treatment are located above each pair of bins (C, control; H, hypoxia; W, wash). Sample size was n = 7 cells for each treatment, asterisk (*) denotes significantly different from normoxia, p < 0.01. In B, fast application of nicotine (10 μm) induced an inward current at −60 mV (holding potential) that was partially inhibited by the α7 nAChR blocker α-btx (100 nm) applied to the same saline-treated (left traces) or nicotine-treated (right traces) cell; the α-btx-sensitive difference current for each of the two cells is shown in the bottom traces.
