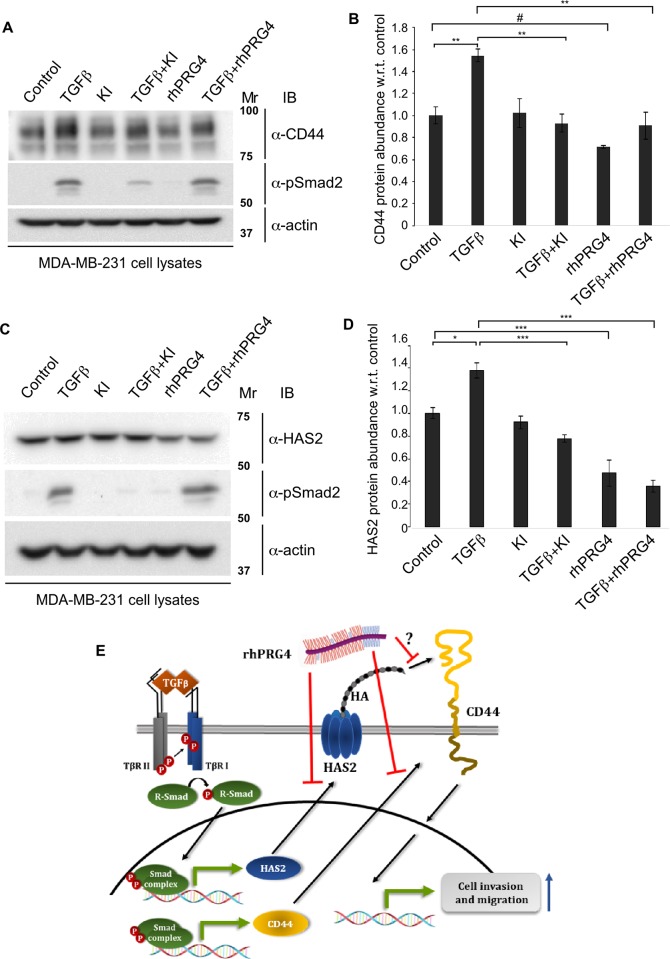
Fig 8. rhPRG4 and TGFβ have opposing effects on the protein abundance of CD44 and HAS2.
(A) CD44 and phospho-Smad2 (pSmad2) immunoblots of lysates of MDA-MB-231 cells incubated in complete growth medium without (control) or with 100 pM TGFβ, alone or together with 10 μM KI or 100 μg/mL rhPRG4. Actin was used as loading control. (B) Bar graph depicts mean ± SEM proportion of CD44 immunoreactive band in each treatment condition from four independent experiments including the one shown in A. (C) HAS2 and phospho-Smad2 (pSmad2) immunoblots of lysates of MDA-MB-231 cells incubated in complete growth medium without (control) or with 100 pM TGFβ, alone or together with 10 μM KI or 100 μg/mL rhPRG4. Actin was used as loading control. (D) Bar graph depicts mean ± SEM proportion of HAS2 immunoreactive band in each treatment condition from four independent experiments including the one shown in D. (E) A schematic diagram showing the relationship amongst TGFβ, HA-CD44 signalling and rhPRG4 in MDA-MB-231 cells, uncovered in this study. TGFβ increases the protein abundance of HAS2 and CD44 to enhance cancer cell’s invasion and migration. rhPRG4 does not alter Smad phosphorylation but decreases HAS2 and CD44 protein abundance leading to suppression of invasion and migration of cancer cells. rhPRG4 may interfere with HA-CD44 interaction. Mr indicates Markers’ molecular size. Significant difference, ANOVA: *P≤ 0.05, **P≤ 0.01, ***P ≤ 0.001;; unpaired T test: #P = 0.0164.