Figure 8. The histone methyltransferase domain of PRDM16 promotes transcriptional silencing.
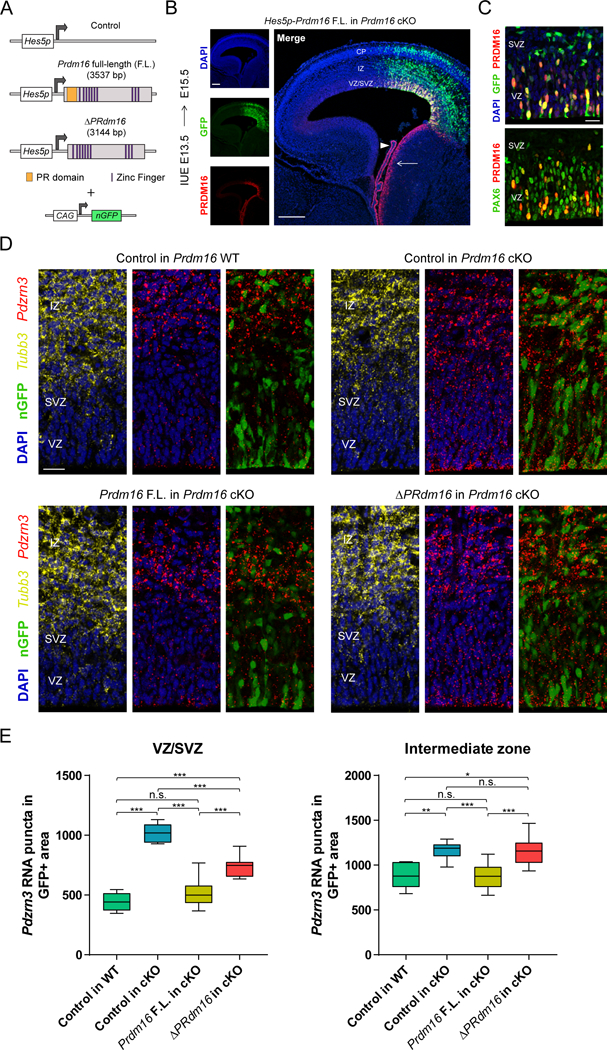
(A) Vectors driving Prdm16 full-length (F.L.) and ΔPRdm16 expression from the Hes5 proximal promoter. The experimental vectors were co-electroporated with a plasmid driving constitutive expression of nuclear GFP.
(B) IUE of Hes5p-Prdm16 F.L. into Prdm16 cKO cortex. Endogenous Prdm16 expression in the choroid plexus (arrowhead) and lateral ganglionic eminence (arrow) are indicated.
(C) IUE of Hes5p-Prdm16 F.L. into E13.5 Prdm16 cKO cortex shows that PRDM16 overlaps with PAX6 in most electroporated cells in the ventricular zone (VZ) and few cells in the subventricular zone (SVZ) at E15.5.
(D) In vivo transcriptional assay of PRDM16 activity in the embryonic cortex. The indicated vectors were electroporated into E13.5 WT or cKO cortex and Pdzrn3 expression in GFP+ cells in the VZ/SVZ and intermediate zone (IZ) was evaluated at E15.5 by fluorescent in situ hybridization. The IZ was identified by Tubb3 expression.
(E) Quantification of Pdzrn3 puncta in GFP+ cells in the cortical VZ/SVZ and IZ of electroporated brains. Results represent number of RNA puncta in 104 μm2 (n=3). Statistical analysis is unpaired Student’s t test (*p < 0.05, **p < 0.01, *** p < 0.001; n.s., not significant). Scale bars: 200 μm (B) and 20 μm (C, D).
