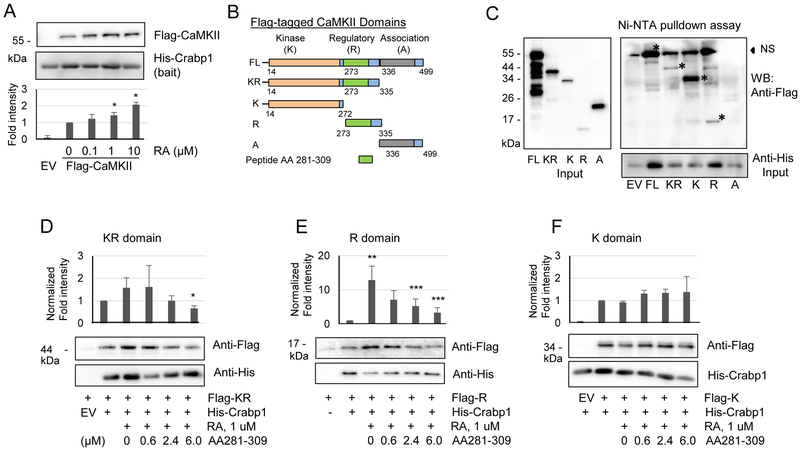
Fig. 5.
RA increases Crabp1 binding to the regulatory domain of CaMKII. (A) RA increases the direct interaction of His-Crabp1 with TnT-generated Flag-CaMKII. Statistical analyses are shown at the bottom. *P < 0.05 vs. control without RA (n=4, student t-test). (B) CaMKII domain map and plasmid constructs (FL, KR, K, R and A) are shown. (C) His-Crabp1 pulldown by Ni-NTA beads co-precipitates Flag-FL, -KR, -K and -R proteins except for Flag-A (the left and bottom panels showing protein input). * signs mark the specific, interacting bands. NS denotes nonspecific bands that slightly overlap with FL. (D-F) Competition assays with CaMKII peptide (residues 281-309) for interaction with KR, R or fragments. The peptide competes with Crabp1 binding to the KR fragment (D) and the R domain (E). (F) The peptide failed to compete with Crabp1 for interaction with K domain. Results show representatives of three independent blots. Statistical analyses are shown above the blot images. *P < 0.05 column 6 vs. column 2, **P < 0.01 column 3 vs. column 2 and **P < 0.05 columns 5 and 6 vs. column 3 (student t-test).