Abstract
Introduction
Patients suffering from chronic kidney disease (CKD) experience a number of associated comorbidities, including anemia. Relative deficiency in renal erythropoietin (EPO) production is thought to be a primary cause of anemia. Interestingly, CKD patients display low levels of hydrogen sulfide (H2S), an endogenously derived renal oxygen sensor. Previous in vitro experiments have revealed that H2S-deficient renal cell lines produce less EPO than wild-type renal cell lines during hypoxia.
Methods
We postulated that H2S might be a primary mediator of EPO synthesis during hypoxia, which was tested using an in vivo murine model of whole-body hypoxia and in clinical samples obtained from CKD patients.
Results
Following a 72-hour period of hypoxia (11% O2), partial H2S knockout mice (lacking the H2S biosynthetic enzyme cystathionine γ-lyase [CSE]) displayed lower levels of hemoglobin, EPO, and cystathionine-β-synthase (CBS) (another H2S biosynthetic enzyme) compared to wild-type mice, all of which was rescued by exogenous H2S supplementation. We also found that anemic CKD patients requiring exogenous EPO exhibited lower urinary thiosulfate levels compared to non-anemic CKD patients of similar CKD classification.
Conclusions
Together, our results confirm an interplay between the actions of H2S during hypoxia and EPO production.
Introduction
Patients with chronic kidney disease (CKD) suffer from a number of associated comorbidities, including the development of anemia, which eventually occurs in almost all end-stage renal disease (ESRD) patients.1 While anemia associated with CKD can be attributed to a variety of factors, relative deficiency in renal erythropoietin (EPO) production is thought to be a primary cause.1 EPO synthesis occurs primarily in the interstitial cells of the peritubular capillaries in response to low oxygen tension, and it goes on to promote erythrogenesis in the bone marrow. CKD patients with anemia are often prescribed erythropoietin-stimulating agents (ESAs), which are associated with a variety of adverse effects, including hypertension and the development of drug resistance.2,3 There is an evident need for novel and alternative ways to treat anemia associated with CKD.
While the correlation between renal failure and development of anemia has been well-established, the exact underlying mechanisms have yet to be confirmed. Patients suffering from CKD have regions of tubular hypoxia due to the progressive interstitial fibrosis that develops around the peritubular capillaries, which are known to sense oxygen tension.2,4,5 This suggests that inadequate EPO production in CKD, despite a state of persistent renal hypoxia, may be due to decreased renal tubular oxygen sensing abilities.4–7 The typical cellular response to hypoxia involves activation of the hypoxia-inducible factor (HIF) pathway to help cells to adapt to reduced oxygen partial pressure that occurs through stabilization of the HIF-α proteins and subsequent transcriptional upregulation of a number of genes, including EPO.5,7–14
In order to allow for an adequate response to low oxygen levels and the initiation of the HIF pathway, cells must be able to sense any changes that may occur in oxygen availability. Current understanding surrounding this process highlights gasotransmitters as oxygen-sensing molecules. There are three gasotransmitters currently believed to act as oxygen sensors: carbon monoxide (CO), nitric oxide (NO), and hydrogen sulfide (H2S). These three gasotransmitters are all believed to fulfill second messenger functions in oxygen sensing.15,16 Emerging evidence suggests that H2S, in particular, may be beneficial during hypoxia, due to interactions with various already well-established hypoxic responses, including the HIF pathway.15,17–20 H2S is endogenously produced in mammalian cells from the metabolism of L-cysteine via the biosynthetic enzymes cystathionine-β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). The resultant H2S is oxidized in the mitochondria to thiosulfates and sulfates. When oxygen levels fall, the mitochondria are unable to catalyze this oxidation, which leads to H2S accumulation that may have a potential role in the mediation of hypoxic responses.15,16,18 Our earlier in vitro work revealed that renal and hepatic cell lines deficient in H2S produce less EPO than their wild-type counterparts during hypoxia.21 Additionally, animal models of CKD demonstrate a decreased ability to produce H2S, which contributes to disease progression.22 This decreased capacity is due to initial downregulation of CBS, which is followed by a decline in both CSE and 3-MST activity. Thus, it is essential to explore whether H2S deficiency plays a role in the inadequate EPO production experienced by CKD patients to further understand the mechanism underlying anemia in CKD.
This study is the first to elucidate the effect of H2S on in vivo renal EPO production. Using an in vivo murine model where mice were subjected to a 72-hour period of hypoxia (11% O2), we discovered that CSE knockout mice displayed lower levels of hemoglobin, EPO, and other HIF-regulated genes compared to wild-type mice. Interestingly, hemoglobin levels and the expression of HIF-regulated genes, EPO, and CBS were rescued by exogenous H2S supplementation. In correlation, we report, for the first time, that anemic CKD patients requiring exogenous EPO exhibited lower urinary thiosulfate levels compared to non-anemic CKD patients of similar CKD classification. This suggests that administering H2S exogenously to anemic CKD patients may potentially have some therapeutic benefit. Overall, our findings highlight the importance of H2S as an oxygen sensor during erythropoiesis and provide evidence that supports the postulate that H2S regulates EPO production through the HIF pathway.
Methods
Animal description and care
Adult male mice (7–8 weeks of age) were housebred and hosted as we previously described.23 Mice were either the wild-type C57BL/6 or were CSE−/− on a C57BL/6 background. We used CSE−/− mice and not CBS−/− mice, as they are not phenotypically viable and several reports have highlighted the preponderance of CSE in renal tissues, including tubules and vasculature, suggesting that it is likely the most pertinent H2S-producing the enzyme for the present study. Also, adult male BDF1 mice (7–8 weeks of age), which are a cross of female C57BL/6 and male DBA/2 and who are commonly used for anemic studies, were purchased from Charles Rivers Laboratories. All conditions were maintained as per SOPs approved by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The experimental protocol followed the guidelines of the Council on Animal Care of our institutions.
Hypoxic and normoxic treatments
Mice were subjected to either normoxia (21% O2, 79% N2) or hypoxia (11% O2, 89% N2) for a 72-hour period in the HypOxystation H85 (Hypoxygen, U.S.). Humidity was maintained at 40%. This period was chosen based on the previously reported time it takes for EPO increase in humans during states of chronic hypoxia. Each strain of mice was assigned to one of the following groups: normoxia, hypoxia + saline, or hypoxia + sodium sulfide (Na2S) injections. Injection of saline (500 uL, intraperitoneal) or sodium sulfide (4 mmol/kg body weight, intraperitoneal) was given twice a day for the 72 hours of hypoxia. After 72 hours, mice were euthanized with CO2, blood was collected via intracardiac blood collection, and all blood samples were sent to London Health Science Core Laboratory, where hemoglobin levels were subsequently measured.
Western blot analysis
A total of 70 μg protein was extracted from the mouse kidneys and was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels. Proteins were transferred onto polyvinylidene difluoride membranes (Roche, Basel, Switzerland) at 100 mV for a 45-minute period. Subsequent to blocking with 5% bovine serum albumin (BSA), membranes were washed and incubated with one of the following primary antibodies: β-actin mouse monoclonal (Santa Cruz Biotechnology Inc., Dallas, TX, U.S.), EPO rabbit polyclonal (GeneTex, Irvine, CA, U.S.), CBS mouse monoclonal (Santa Cruz Biotechnology Inc., Dallas, TX, U.S.), and NfkB-p65 rabbit monoclonal (GeneTex, Irvine, CA, U.S.). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, PA, U.S.) and developed using the LI-COR C-DigitTM Blot Scanner (Mandel Scientific Company INC, ON, Canada). Bands were quantified using the LI-COR Image Studio Software.
Whole blood H2S level measurements
Following euthanasia in a CO2 chamber, intracardiac blood collection was performed on all mice; 100 mL of whole blood was collected and placed in a cryovial already containing 100 mL of the antioxidant buffer solution, which was prepared fresh weekly by the Lazar Research Laboratories protocol for the Micro Sulfide Ion Electrode (Model LIS-146AGSCM). Due to the presumed short half-life of H2S, all measurements were performed within five minutes of euthanasia. Whole blood H2S levels were determined through comparison to a standard curve of sodium sulfide solutions that was again prepared by the Lazar Research Laboratories protocol for the Micro Sulfide Ion Electrode (Model LIS-146AGSCM). These levels obtained were an approximation, and meant to determine a difference between groups based on relative differences. They are not meant to give exact whole blood H2S levels.
CKD and healthy control patient eligibility criteria
This patient population was chosen to determine whether CKD patients who develop EPO-deficient anemia have lower urinary thiosulfate levels than their non-anemic counterparts. Eligibility criteria consisted of the patient being over the age of 18 and having a glomerular filtration rate (GFR) between 10 and 19 mL/min. Additionally, the patient had to attend regular treatments or assessments for CKD. Exclusion criteria included patients who: did not have CKD, had previous renal transplants, had recurrent urinary tract infections (UTIs), or had gross hematuria. Patient medical histories were evaluated to obtain relevant medical information, and the values outlined in Table 1 are taken from their medical records around the time of the clinic visit that they provided the urine sample. Both groups (CKD ESA+ and CKD ESA-) were matched for age and GFR prior to comparison of urinary thiosulfate levels.
Table 1.
Characteristics of study participants. Baseline characteristics of CKD patients who were either on ESA or not
| ESA+ | ESA− | p | |
|---|---|---|---|
| Age (y) | 70.6±14.7 | 65.7±14.4 | 0.43 |
| Gender | |||
| Male | 7 | 7 | |
| Female | 2 | 3 | |
| Hemoglobin (g/dL) | 106.2±11.5 | 113.6±9.9 | 0.1888 |
| eGFR | 13.8±2.0 | 15.8±3.1 | 0.1274 |
| BUN (mmol/L) | 22.9±9.5 | 20.7±8.5 | 0.2407 |
| Calcium (mmol/L) | 2.1±0.1 | 2.1±0.3 | 0.2868 |
| Type of ESA | |||
| Epoetin alfa | 3 | 0 | |
| Darbepoetin alfa | 6 | 0 | |
| Dose of ESA | |||
| Epoetin alfa (units) | 3333±63.3 | N/A | |
| Darbepoetin alfa (μg/month) | 63.33±46.76 | N/A | |
Values are expressed as mean ± standard deviation. BUN: blood urea nitrogen; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ESA: erythrocyte stimulating agents.
CKD patient urine collection and analysis of urinary thiosulfate levels
Patients who consented were asked to provide a urine sample at their regular clinic visit. Due to the presumed short half-life of H2S, samples were immediately aliquoted into four cryovials and snap frozen with liquid nitrogen spray within 10 minutes of the sample being provided. Samples were stored at −80°C, with any transport of samples being completed on dry ice. Urinary thiosulfate levels were analyzed using a specific HPLC method described by Farese et al.24 Urinary sulfate levels were determined chromatographically (type 861; Metrohm, Herisau, Switzerland).
Statistical analysis
All results were analyzed using one-way ANOVA, Mann Whitney-U tests, or Kruskal-Wallis tests wherever appropriate. Significance was accepted at the 95% confidence interval (CI). Statistical significance was defined as a p<0.05. Data were represented as mean ± standard deviation (SD) unless indicated otherwise.
Study approval
Approval from the Western University Health Sciences Research Ethics Board (REB) was sought to complete the study involving CKD patients, as well as the study involving the animal experiments. Patients provided us with written informed consent before they were included in the study. The experimental protocol for animals followed the guidelines of the Council on Animal Care of our institutions.
Results
CSE−/− mice exhibit decreased hemoglobin levels during hypoxia compared to wild-type mice
To further strengthen the link between H2S and the hypoxia-induced erythrogenic response, wild-type and CSE−/− mice were placed in either normoxia (21% O2) or hypoxia (11% O2) for 72 hours. During this period, mice in hypoxia were subjected to two injections of either saline or the H2S-releasing molecule Na2S (4 mmol/kg body weight) daily. Blood was collected at the time of sacrifice at the end of the 72-hour period, and hemoglobin levels were determined. Hypoxic wild-type mice displayed a significant increase in hemoglobin levels (Fig. 1) as compared to normoxic wild-type mice, which was to be expected. In comparison, hypoxic CSE−/− mice displayed significantly lower hemoglobin levels compared to hypoxic wild-type mice. CSE−/− mice have previously been reported to produce as much as 70% less H2S than wild-type mice.23 When exogenous H2S (Na2S) was given to CSE−/− mice during hypoxia, hemoglobin levels were significantly rescued and comparable to hemoglobin levels of normoxic wild-type and CSE−/− mice, which suggests that H2S appears to play an important role when endogenous production is muted.
Fig. 1.
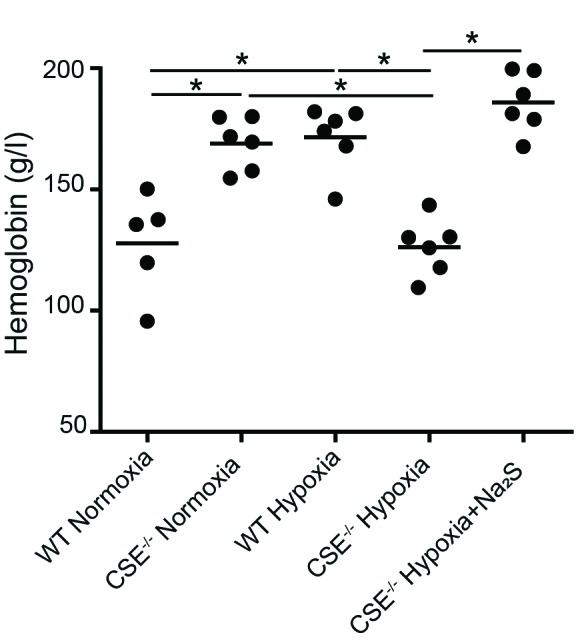
Hemoglobin levels in wild type and CSE−/− mice following varying oxygen conditions. Wild type (WT) and CSE−/− mice were either sacrificed under normoxic conditions, or placed in hypoxia (11% O2) for 72 hours prior to sacrifice with twice-daily injections of saline or Na2S. Blood was collected via cardiac puncture at time of sacrifice, and hemoglobin was measured. Values are expressed as mean ± standard deviation. *p<0.05.
Whole blood H2S levels are decreased in CSE−/− mice during hypoxia compared to wild-type mice
While the whole blood H2S levels in both CSE−/− and wildtype mice have been previously documented,23 we demonstrate the first temporal relationship between whole blood H2S levels during hypoxia. Whole blood H2S levels were measured in blood collected following euthanasia using a sulfide/H2S-sensitive microelectrode system. Although the electrode is not designed to detect absolute levels, it provides relative measurements accurately and reliably, and that are reflective of differences in whole blood H2S levels between groups. When comparing whole blood H2S levels of hypoxic and normoxic mice from the same mouse strains, there were no detectable differences for either the wild-type or the CSE−/− mice (Fig. 2). CSE−/− mice injected twice daily with Na2S during the 72-hour period of hypoxia exhibited a significant increase in whole blood H2S levels, which were comparable to H2S levels detected in the blood of wild-type animals. In normoxic conditions, wild-type mice exhibited significantly higher whole blood H2S levels than CSE−/− mice. These findings complement our observations about hemoglobin levels and suggest that endogenous H2S production may be an essential modulator of oxygen sensing and response to hypoxic environments.
Fig. 2.
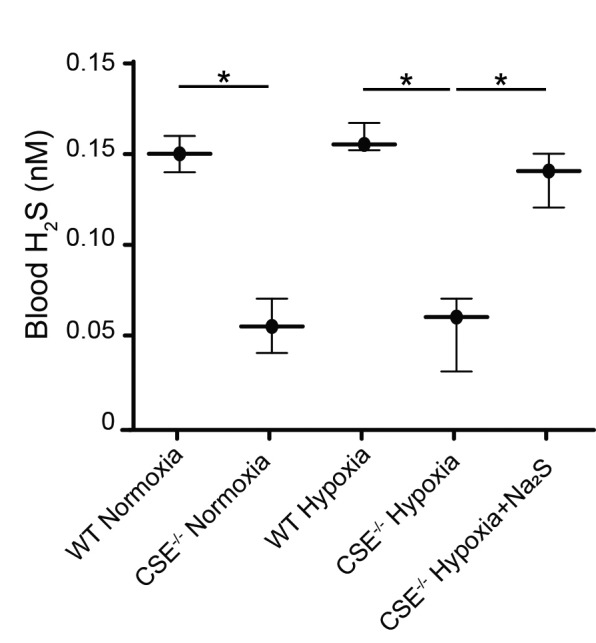
Whole blood H2S levels in wild type and CSE−/− mice following varying oxygen conditions. Wild type (WT) and CSE−/− mice were either sacrificed under normoxic conditions, or placed in hypoxia (11% O2) for 72 hours prior to sacrifice with twice-daily injections of saline or Na2S. Blood was collected via cardiac puncture at time of sacrifice, and H2S level were measured using a H2S electrode. Values are expressed as mean ± standard deviation. *p<0.05.
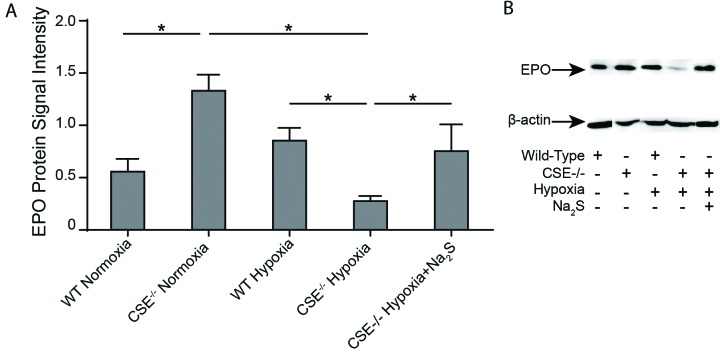
CSE−/− mice exhibit decreased EPO production during hypoxia compared to wild-type mice
Although there was an observable increase in EPO levels in wild-type mice following 72 hours of hypoxia, this change was not statistically significant (Fig. 3). CSE−/− mice exhibited significantly higher EPO levels than wild-type mice during normoxia. During hypoxia, this trend was reversed, and wild-type mice exhibited a significantly higher level of EPO production than their CSE−/− counterparts. Interestingly, the significant decline in EPO production following hypoxia in CSE−/− mice was rescued in the CSE−/− mice treated with twice-daily injections of Na2S during the hypoxic period. These results are consistent with our observations about hemoglobin levels (Fig. 1). CSE−/− mice exhibited impaired EPO production and hemoglobin levels during hypoxia, which indicates that the effects of H2S on EPO and, thus, hemoglobin levels differ depending on oxygen availability. In clinical states where endogenous H2S levels are decreased, such as the relative tissue hypoxia that is observed in CKD patients who develop intrarenal fibrosis, these findings reveal an invaluable opportunity for the development of novel therapeutics.
Fig. 3.
Erythropoietin levels in wild type and CSE−/− mice following varying oxygen conditions. Wild type (WT) and CSE−/− mice were either sacrificed under normoxic conditions, or placed in hypoxia (11% O2) for 72 hours prior to sacrifice with twice-daily injections of saline or Na2S. Kidneys were harvested and frozen at time of sacrifice. Western blots were performed using protein extracted from mouse renal tissue in order to determine erythropoietin (EPO) levels in the various mouse groups. n=3. Values are mean ± standard deviation. *p<0.05.
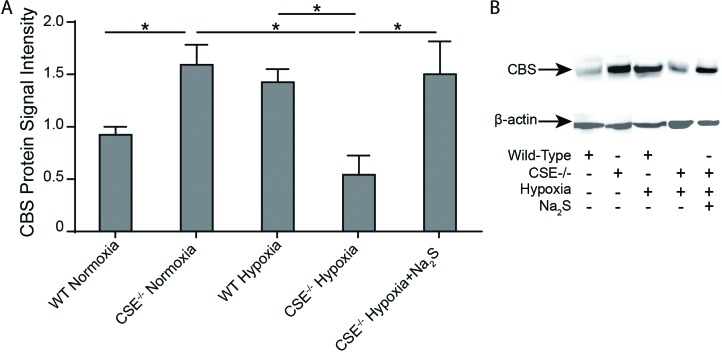
CSE−/− mice exhibit decreased CBS levels during hypoxia as compared to wild-type mice
Given that the H2S biosynthetic enzyme CBS has also been reported to be regulated by the HIF pathway,25 we evaluated its potential role in the hypoxic response. Interestingly, CBS levels were upregulated in the renal tissue of CSE−/− mice as compared to wild-type mice during normoxia (Fig. 4), which suggests that CBS expression is constitutively enhanced due to lack of CSE expression. While CBS protein levels did not change in wild-type animals following 72 hours of hypoxia, CBS protein levels significantly decreased in CSE−/− mice in response to 72 hours of hypoxia, which indicates that hypoxia downregulates CBS expression. Additionally, CBS protein levels in hypoxic CSE−/− mice treated with Na2S injections were comparable to CBS protein levels in normoxic CSE−/− mice, and were significantly higher than CBS protein levels in untreated hypoxic CSE−/− mice. The increased CBS protein levels in hypoxic CSE−/− mice that received Na2S injections likely contributes to the increased whole blood H2S levels previously observed in this group of mice in addition to the exogenous H2S supplementation (Fig. 2). Taken together with the previous observations, these results suggest that CBS expressed in the kidneys of CSE−/− mice is unable to compensate for the lack of CSE expression during hypoxic conditions despite constitutive upregulation in normoxic conditions.
Fig. 4.
Cystathionine-β-synthase (CBS) levels in wild type and CSE−/− mice under varying oxygen conditions. Wild type (WT) and CSE−/− mice were either sacrificed under normoxic conditions, or placed in hypoxia (11% O2) for 72 hours prior to sacrifice with twice-daily injections of saline or Na2S. Kidneys were harvested and frozen at time of sacrifice. Western blots were performed using protein extracted from mouse renal tissue in order to determine CBS levels in the various mouse groups. n=3. Values are mean ± standard deviation. *p<0.05.
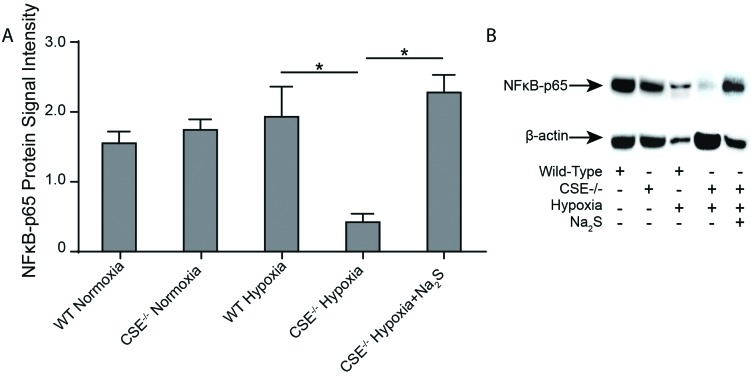
CSE−/− mice exhibited decreased NFκB-p65 levels during hypoxia compared to wild-type mice
Previous research has identified NFκB-p65 as a transcriptional regulator of the HIF pathway.26–28 Therefore, we chose to examine NFκB-p65 protein levels in our various treatment groups to determine whether this could be the point of action for H2S’s influence on EPO production.26–28 There were no significant differences found in NFκB-p65 between CSE−/− mice and wild-type mice during normoxia. Although there was no significant change in NFκB-p65 levels observed in wild-type mice following 72 hours of hypoxia, CSE−/− mice demonstrated a significant decrease in NFκB-p65 protein levels following the hypoxia period. This phenomenon was reversed entirely if Na2S was administered to CSE−/− mice during the hypoxic period (Fig. 5). These results suggest that H2S may exert its influence on the HIF pathway and, thus, erythrogenesis through interaction with NFκB-p65.
Fig. 5.
NFκB-p65 levels in wild type and CSE−/− mice under varying oxygen conditions. Wild type (WT) and CSE−/− mice were either sacrificed under normoxic conditions, or placed in hypoxia (11% O2) for 72 hours prior to sacrifice with twice-daily injections of saline or Na2S. Kidneys were harvested and frozen at time of sacrifice. Western blots were performed using protein extracted from mouse renal tissue in order to determine NFκB -p65 levels in the various groups. n=3. Values are mean ± standard deviation. *p<0.05.
Sodium sulfide injections reduce hemoglobin levels in BDF1 mice during normoxia
In order to better determine the role of H2S during normoxic conditions, Na2S injections were given to BDF1 mice (Fig. 6), which are a cross between C57BL/6 and DBA/2. These mice exhibit normal erythrogenesis during normoxia and are commonly used in EPO research. Therefore, they were chosen as an additional wild-type control.29 These mice were placed in normoxia and given either saline or Na2S injections twice daily for a 72-hour period or Na2S injections twice daily for an extended 120-hour period. Animals given Na2S injections for the extended period experienced significantly reduced hemoglobin levels as compared to those given either saline or Na2S for a 72-hour period, suggesting that excess H2S may be detrimental to erythrogenesis during normoxia.
Fig. 6.
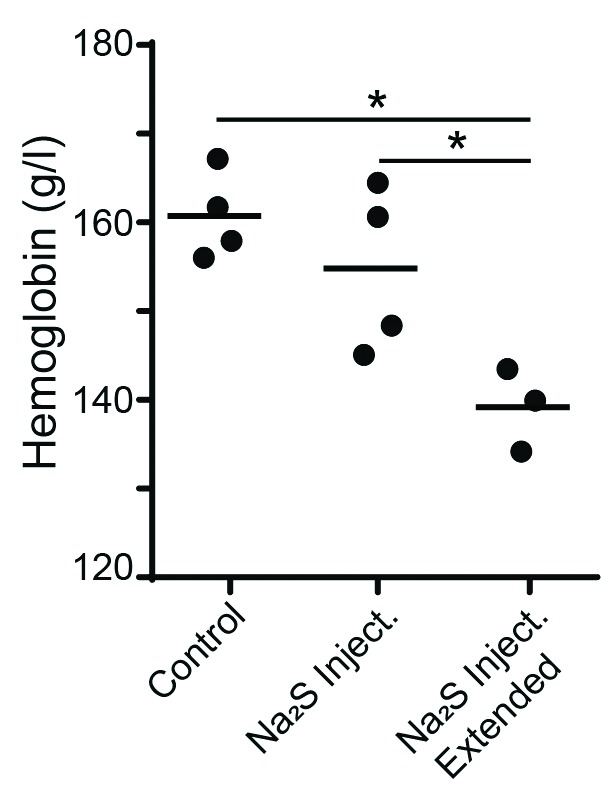
Hemoglobin levels in BDF1 mice under varying oxygen conditions. Daily Na2S injections reduce hemoglobin levels in BDF1 mice during normoxia. BDF1 mice were placed in normoxia and administered either saline injections for a period of 72 hours, Na2S injections twice daily for a period of 72 hours or Na2S injections twice daily for a period of 120 hours. n= 3 and 4. Values are mean ± standard deviation. *p<0.05.
Urinary thiosulfate levels are lower in anemic CKD patients than non-anemic CKD patients
Thiosulfate is a metabolite of H2S and is believed to play an essential part in the oxygen-sensing role of H2S. Thiosulfate levels present in the urine of CKD patients were measured using HPLC.30 Additionally, patient urinary creatinine levels were examined to ensure comparable concentration of urine. Characteristics of patients who participated in the study are highlighted in Tables 1 and 2. Patients who met the eligibility criteria were separated based on whether or not they currently required the use of the ESAs (epoetin alfa and darbepoetin alfa). In comparison to CKD patients who do not develop anemia, CKD patients who require the use of ESAs exhibit significantly lower urinary thiosulfate levels (Fig. 7A). The urinary thiosulfate levels were normalized relative to urinary creatinine levels. Additionally, the estimated (e)GFR of both patient groups was matched in order to ensure comparison of patients with similar kidney function and severity of CKD (Fig. 7B). There was no significant difference in urinary thiosulfate levels when patients were divided based on gender, smoking status, or whether they were diabetic. Interestingly, we found that measuring urine H2S levels using an electrode meter gave similar trends in arbitrary units of H2S (data not shown). Notably, urinary thiosulfate and H2S levels in normal healthy volunteers were 3.705 mM±3.66, which was significantly higher than levels measured in both groups of patients with CKD.
Table 2.
Original causes for CKD in patients who are either ESA+ (anemic) or ESA− (non-anemic)
| Cause of CKD | ESA+ | ESA− |
|---|---|---|
| Diabetes mellitus | 6 | 2 |
| Hypertension | 3 | 2 |
| Polycystic kidney disease | 0 | 4 |
| Dense deposit disease | 1 | 0 |
| Renovascular disease | 1 | 1 |
| Reflux nephropathy | 0 | 1 |
| FSGS | 0 | 0 |
| Unknown | 0 | 0 |
| Amyloidosis | 1 | 0 |
| Obstructive uropathy | 0 | 0 |
| Solitary kidney | 0 | 0 |
| Resistant membranous glomerulonephritis | 0 | 0 |
| Nephrocalcinosis | 0 | 0 |
| Chronic NSAID use | 0 | 2 |
CKS: chronic kidney disease; ESA: erythropoietin-stimulating agents; NSAID: non-steroidal anti-inflammatory drug.
Fig. 7.
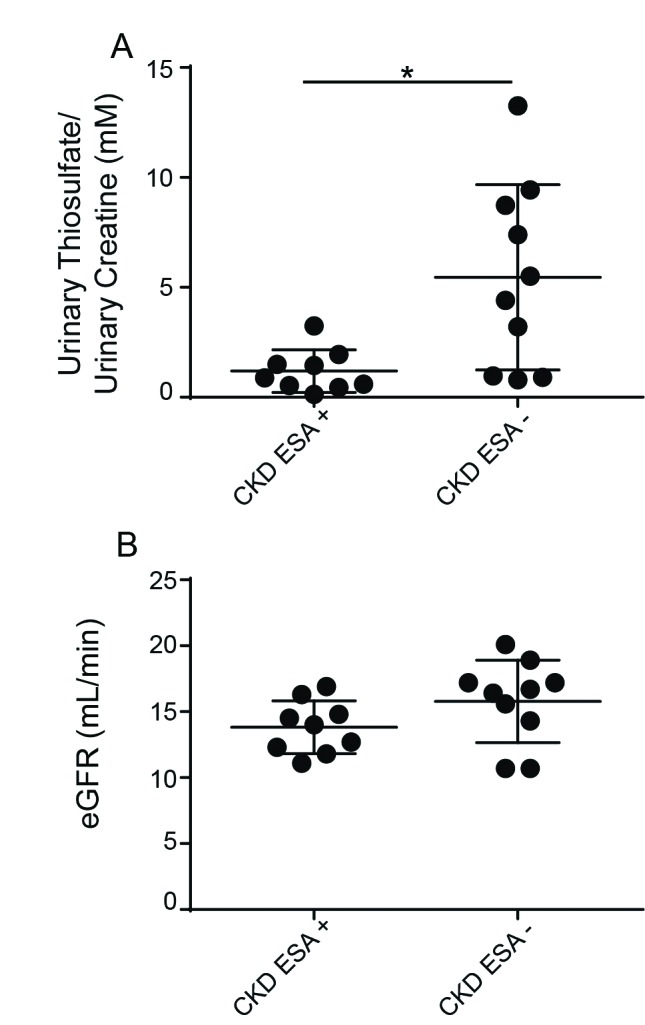
Urinary thiosulfate levels are diminished in anemic chronic kidney disease (CKD) patients compared to non-anemic CKD patients. Urine samples were collected from CKD patients, aliquoted and flash frozen to −80°C. (A) Urinary thiosulfate levels were measured using HPLC and normalized relative to urinary creatinine levels and urine samples from patients requiring erythrocyte-stimulating agents (ESA) for anemia of CKD showed lower urinary thiosulfate levels. (B) Estimated glomerular filtration rate (eGFR) was matched between both groups to ensure comparison of patients with similar kidney function and severity of CKD. Values are mean ± standard deviation. *p<0.05.
Discussion
We have previously demonstrated, in an in vitro setting that H2S supplementation has a significant positive impact on EPO production during hypoxia but not normoxia.21 The current study is the first to demonstrate that this phenomenon is preserved both in animal and human models. Typically, during hypoxia, the body responds by increasing the amount of EPO that is produced, which eventually results in increased hemoglobin levels.5 We expected that both wild-type and CSE−/− mice would exhibit increased hemoglobin levels during hypoxia as compared to normoxia. This phenomenon remained true for wild-type mice, however, hypoxic CSE−/− mice interestingly exhibited lower hemoglobin levels than their normoxic counterparts. In combination with other results from this study, this suggests that H2S is both needed and beneficial during hypoxia in order to produce this hypoxic response. Interestingly, excess H2S was found to decrease hemoglobin levels in normal mice during normoxia, suggesting that high levels of H2S may, in fact, be detrimental to erythrogenesis in a resting state. This indicates that the role of H2S in erythrogenesis is likely dependent on oxygen availability, thus resulting in differing effects of exogenous H2S treatment observed during normoxia and hypoxia. We also demonstrate, for the first time, that CKD patients have lower urinary H2S levels compared to non-CKD patients, and that patients who require ESAs for anemia associated with CKD produce even less urinary thiosulfates (an active metabolite of H2S) compared to non-anemic CKD patients who do not require ESAs. This further strengthens the argument that H2S is an essential modulator of erythrogenesis, and that this phenomenon may be intrinsic to the kidney.
Previous studies have demonstrated that genetic knockout of CSE results in substantial decreases in endogenous H2S levels, despite it being only one of three H2S producing enzymes,23 which was consistent with our findings. Additionally, our previous in vitro data had revealed that CBS, which is the other major HIF-regulated H2S producing the enzyme in renal tissue, was affected by H2S and O2 availability.22 We chose not to measure levels of the other H2S biosynthetic enzyme mercaptopyruvate sulfurtransferase (MPST), as it has not been shown to be HIF-regulated and we specifically looked at HIF-regulated genes and pathways in this study. When we examined CBS protein expression in the mouse groups, CBS levels were found to be significantly upregulated in CSE−/− mice during normoxia as compared to wild-type. While this upregulation is likely in response to the lack of CSE activity, it remains insufficient to completely compensate for CSE deficiency, as CSE−/− mice exhibit decreased endogenous whole blood sulfide/H2S levels. However, it is possible that CBS upregulation could affect intrarenal H2S levels and more locally compensate for the loss of CSE-mediated H2S production. During hypoxia, the opposite trend exists, where CSE−/− mice exhibited decreased CBS levels as compared to wild-type; however, despite this downregulation, there was not a significant decrease in blood H2S levels between hypoxia and normoxia, which may suggest that CBS is not be as crucial in EPO production and anemic response to hypoxia as CSE is. Also, it is important to restate that typically, there is a more significant amount of H2S present during normoxia than hypoxia due to the rapid oxidation by the mitochondria, which occurs when normal oxygen levels are available. With this in mind, it is possible that there exists a higher amount of H2S/sulfide in the normoxic CSE−/− mice, but we were unable to measure it accurately due to its rapid oxidation. Again, the values of blood H2S presented in this study are not representative of an actual amount but are used to determine relative differences between groups.
While the majority of research concerning the HIF pathway has revealed that it is primarily regulated post-translationally, some studies have demonstrated that it can also be regulated transcriptionally by NfκB-p65.27 Our previous in vitro experiments found that H2S influenced HIF-1α and HIF-2α at the transcriptional level, indicating that its influence was likely due to an interaction with an upstream HIF transcriptional regulator.22 Additionally, our previous work found that H2S likely interacts with HIF-1α and HIF-2α differently, and thus regulated their respective downstream genes differently. This could potentially explain why VEGF, a HIF-1α regulated gene, did not follow the same upregulation/downregulation pattern (data not shown) as HIF-2α-regulated genes such as EPO. Seeing as CBS, however, did follow a similar pattern to EPO, it is possible that CBS is regulated by the HIF-2 pathway. Lastly, downregulation of NFκB-p65 coincides with downregulation of the HIF-regulated genes EPO and CBS, which is consistent with findings from previous studies that downregulation of NFκB-p65 during hypoxia also results in downregulation of some HIF-regulated genes.28,29 Taken together, this indicates that the influence H2S exerts on HIF-regulated genes could be through its interaction with NfκB-p65. Further research with this in vivo model using greater sample sizes is required to determine the exact mechanisms and interactions between H2S and the HIF pathway, which should also elucidate the effects of H2S on HIF-1α and HIF-2α individually.
Considering the overall trends seen from the abovementioned in vitro study, it is likely that H2S plays dual roles depending upon oxygen conditions: the first is during hypoxia, where it stimulates HIF pathways and downstream genes; and the second is during normoxia, where it decreases the activity of HIF downstream genes. This could potentially explain why the CS −/− mice have less EPO, hemoglobin, and CBS following hypoxia than normoxia. Additionally, despite the knockdown of CSE in these mice, the MPST/CBS H2S-producing pathways are still intact, making it attractive that these trends were so apparent. CSE−/− mice are thought to have up to a 70% reduction in total H2S synthesis.25 Perhaps even though MPST and CBS are still intact, these systems are not as involved with erythropoiesis, or they are just unable to compensate for this drastic reduction in H2S production. Another possibility is that there is increased hemolysis or hemorrhage in these mice, although, we did not see evidence of hemolysis, as there was no evidence of splenomegaly or spherocytosis on the blood smears. We evaluated for hemorrhage post-mortem and found no evidence. The mouse red blood cell half-life is approximately eight days, and thus we evaluated for reticulocytosis and found a reduction in these with hypoxia, so it is possible that CSE may be the most critical H2S enzyme involved in hematopoiesis. In fact, this reduction in reticulocytes was increased when H2S was added. Further research is needed to sort out this phenomenon.
Clinical studies examining H2S levels in CKD patients have revealed abnormally low plasma H2S levels present in a significant portion of these individuals.24,31,32 In particular, patients undergoing hemodialysis demonstrate significantly reduced levels, which coincide with decreased CSE expression.24 Based on this knowledge and our findings mentioned above that decreased H2S production yields decreased EPO production, we chose to examine urinary thiosulfate levels in CKD patients, as this is believed to be reflective of the H2S that is produced by the kidneys. Patients who had both EPO-deficient anemia and CKD exhibited significantly lower urinary thiosulfate levels than patients with CKD alone, which supports our model of H2S being essential for hypoxia-induced EPO production. In fact, we measured urinary H2S levels using a commercially available electrode-based detection system concomitantly, which also showed a statistically significant difference between the two groups, as well as a significant difference between CKD patients and healthy controls; these results were not reported in the current study. Given that the gasotransmitter NO has previously been shown to regulate EPO production, we examined the levels of urinary nitrates and nitrites in these patients to rule out this effect. Although dietary intake of nitrates was not measured, we found no differences in the urinary nitrate and nitrite levels (μM) between the anemic (nitrite=0.12, nitrate=347.90) and non-anemic (nitrite=0.21, nitrate=250.93) patient populations in our study (p>0.05). Given that these were clinic patient samples, we did not measure serum H2S and NO levels.
Taken together, the results of this human study are promising and indicate two key findings. The first key finding is that anemic CKD patients have lower urinary thiosulfate levels than CKD patients who do not suffer from EPO-deficient anemia. The second key finding is that the analysis of spot urine samples using a commercially available H2S probe may be used in clinical settings, not as an absolute measure of urine levels but rather to determine relative values compared to healthy subjects.
Conclusions
Our study is the first to use both animal and clinical data to highlight the postulated interaction between H2S and the HIF pathway to regulate the production of HIF target genes during hypoxia (Fig. 8). Knocking out one of the three major H2S biosynthetic enzymes results in significant reductions in hemoglobin, EPO, CBS, and NFκB-p65 levels as compared to wild-type mice during hypoxia, which can be reversed upon exogenous H2S supplementation. During normoxia, this phenomenon is reversed, as hemoglobin and a variety of HIF-regulated genes are significantly upregulated as compared to wild-type mice. Lastly, these results are similar to findings from a clinical setting, as anemic CKD patients have significantly lower urinary thiosulfate levels than CKD patients who do not develop anemia, thus demonstrating the importance of H2S for red blood cell levels at both the murine and clinical scenarios.
Fig. 8.
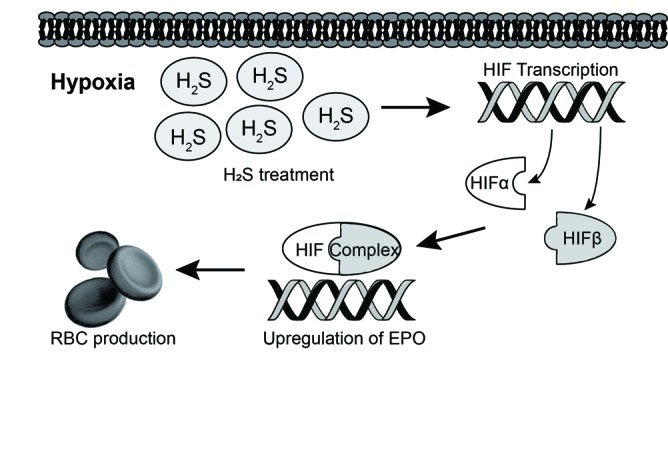
Model of H2S-mediated erythropoietin (EPO) stimulation through the HIF pathway. The above figure represents our proposed model of how H2S stimulates the production of EPO and other HIF-regulated genes. H2S treatment during hypoxia leads to increased transcription of the HIF-2α subunit through NFκB-p65. This leads to increase levels of the HIF dimer, which goes on to bind to various HIF-regulated genes. Transcription of these HIF-regulated genes, including EPO, is then increased, which eventually results in increased erythrogenesis and, therefore, increased red blood cell production. As demonstrated in our data using animal and clinical samples, when H2S production is blunted, likely as a result of interstitial fibrosis of progressive CKD, these proposed pathways are dysregulated and hence contribute to decreased erythrogenesis. This may be a novel potential avenue for therapeutic development.
Overall, these findings indicate a previously undocumented interaction between H2S and the HIF pathway in stimulating EPO and other HIF-regulated gene products in states of hypoxia. Additionally, we have uncovered a previously unexplored role of H2S in the regulation of HIF-regulated genes during normoxic conditions, thus further highlighting the role of H2S as an oxygen sensor. These results provide greater insight into the regulation of the HIF pathway and should be further explored for the development of potential therapeutic strategies. Additionally, the results presented in this study pave the way for the potential development of orally bioavailable supplemental H2S donor molecules for clinical use in patients who exhibit low endogenous levels of H2S. Exogenous supplementation of H2S could one day represent a more efficacious, cost-effective, and better-tolerated alternative to standard injectable therapies in the treatment of anemia associated with CKD, resulting in increased quality of life for CKD patients.
Footnotes
Competing interests: Dr. Pasch has received an unrestricted research grant from Köhler Chemie, a provider of sodium thiosulfate. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
Funding: This study was primarily funded by the Lawson Health Research Grant. Additionally, Dr. Rui Wang is supported by CIHR.
References
- 1.Eckardt K-U. Anaemia in end-stage renal disease: Pathophysiological considerations. Nephrol Dial Transplant. 2001;16:2–8. doi: 10.1093/ndt/16.suppl_7.2. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y-T, Pan S-Y, Lin S-L. Seeking for a way to revive erythropoietin production in chronic kidney disease. J Formos Med Assoc. 2013;112:657–8. doi: 10.1016/j.jfma.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Hung S-C, Lin Y-P, Tarng D-C. Erythropoiesis-stimulating agents in chronic kidney disease: What have we learned in 25 years? J Formos Med Assoc. 2014;113:3–10. doi: 10.1016/j.jfma.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami T, Mimura I, Shoji K, et al. Hypoxia and fibrosis in chronic kidney disease: Crossing at pericytes. Kidney Int Suppl. 2014;4:107–12. doi: 10.1038/kisup.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3:a011619–a011619. doi: 10.1101/cshperspect.a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol. 2013;24:537–41. doi: 10.1681/ASN.2012080855. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JW. Erythropoietin: Physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Gai JW, Wang Y, et al. Characterization of hydrogen sulfide and its synthases, cystathionine betasynthase, and cystathionine gamma-lyase in human prostatic tissue and cells. Urology. 2012;79:483.e1–5. doi: 10.1016/j.urology.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Kapitsinou PP, Liu Q, Unger TL, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–48. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke K, Gassmann M, Wielockx B. Erythrocytosis: The HIF pathway in control. Blood. 2013;122:1122–8. doi: 10.1182/blood-2013-01-478065. [DOI] [PubMed] [Google Scholar]
- 11.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Physiol. 2010;299:F1–13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R. Gasotransmitters: Growing pains and joys. Trends Biochem Sci. 2014;39:227–32. doi: 10.1016/j.tibs.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Lobb I, Sonke E, Aboalsamh G, et al. Hydrogen sulphide and the kidney: Important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide. 2015;46:55–65. doi: 10.1016/j.niox.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Lobb I, Sonke E, Aboalsamh G, et al. Hydrogen sulphide and the kidney: Important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide Biol Chem. 2015;46:55–65. doi: 10.1016/j.niox.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H. Hydrogen sulfide: Its production, release, and functions. Amino Acids. 2010;41:113–21. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 19.Olson KR. Hydrogen Sulfide and its Therapeutic Applications. Springer; Vienna: 2013. Hydrogen sulfide as an oxygen sensor [Internet] pp. 37–62. [DOI] [Google Scholar]
- 20.Budde MW, Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol Biol Cell. 2010;21:212–7. doi: 10.1091/mbc.e09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannigan KL, Agbor TA, Motta J-P, et al. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 2015;29:1591–602. doi: 10.1096/fj.14-266015. [DOI] [PubMed] [Google Scholar]
- 22.Leigh J, Saha MN, Mok A, et al. Hydrogen sulfide-induced erythropoietin synthesis is regulated by HIF proteins. J Urol. 2016;196:251–60. doi: 10.1016/j.juro.2016.01.113. [DOI] [PubMed] [Google Scholar]
- 23.Aminzadeh MA, Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant. 2011;27:498–504. doi: 10.1093/ndt/gfr560. [DOI] [PubMed] [Google Scholar]
- 24.Farese S, Stauffer E, Kalicki R, et al. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol. 2011;6:1447–55. doi: 10.2215/CJN.10241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine-lyase. Science. 2008;322:587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandarra D, Rocha S. A tale of two transcription factors: NF-kB and HIF crosstalk. OA Mol Cell Biol. 2013;1 doi: 10.13172/2054-7331-1-1-924. [DOI] [Google Scholar]
- 28.Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung Y-J, Isaacs J-S, Lee S, et al. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–7. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 30.Pandey SK, Kim K-H, Tang K-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal Chem. 2012;32:87–99. doi: 10.1016/j.trac.2011.08.008. [DOI] [Google Scholar]
- 31.Sen N, Paul BD, Gadalla MM, et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perna AF, Luciano MG, Ingrosso D, et al. Hydrogen sulfide, the third gaseous signaling molecule with cardiovascular properties, is decreased in hemodialysis patients. J Ren Nutr. 2010;20:S11–4. doi: 10.1053/j.jrn.2010.05.004. [DOI] [PubMed] [Google Scholar]