Fig. 2.
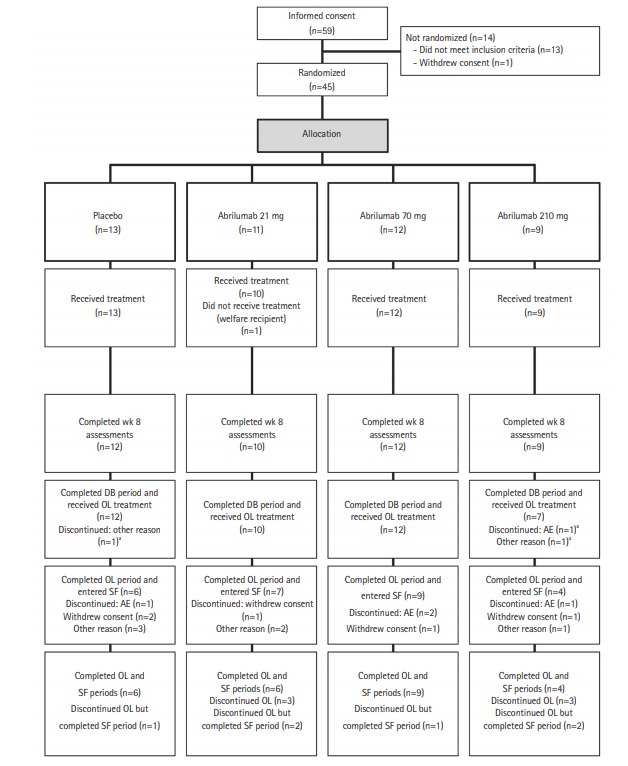
Patient disposition. aThe 3 patients who discontinued the double-blind (DB) period all completed the safety follow-up (SF) period. OL, open-label; AE, adverse event.

Patient disposition. aThe 3 patients who discontinued the double-blind (DB) period all completed the safety follow-up (SF) period. OL, open-label; AE, adverse event.