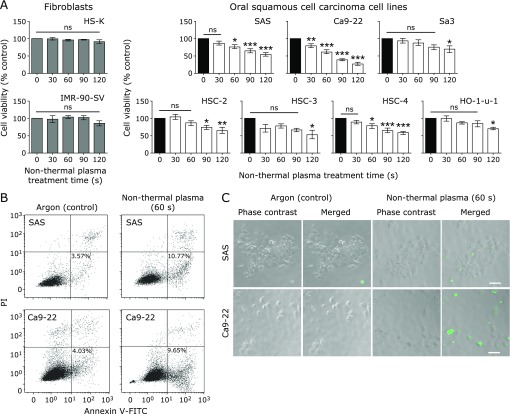
Fig. 1.
Cell viability assay and detection of apoptosis. (A) Cell viability assay. Nine cell lines were cultured in 96-well plates at 5,000 cells/well for 24 h at 37°C. Non-thermal plasma (NTP) (30, 60, 90, and 120 s at 25°C) was applied, Argon (Ar) alone was applied for 60 s to the control cells, and the cells were cultured for 24 h at 37°C. Absorbance was measured using the WST-8 cell proliferation assay and POWERSCAN 4 microtiter plate reader. Error bars represent the standard error of the mean. *p<0.05, **p<0.01, ***p<0.001; ns, not significant. (B) Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) analysis. In the NTP group and the Ar alone group, a total of 1.5 × 106 cells/well were seeded in a 60-mm dish and cultured for 24 h at 37°C. NTP or Ar alone was applied, and cells were incubated for 24 h at 37°C. After incubation, apoptosis was measured using flow cytometry. (C) Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. In the NTP group and the Ar alone group, a total of 5.0 × 103 cells/well were seeded in a 96-well plate and cultured for 24 h at 37°C. NTP or Ar alone was applied, and cells were incubated for 24 h at 37°C. After incubation, a TUNEL assay was performed. Scale bar = 50 µm.