Table 1.
Development of the catalytic system for direct asymmetric conjugate addition of EtMgBr to carboxylic acid 1aa
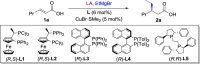
| ||||||
|---|---|---|---|---|---|---|
| Entry | L/Cu(I) | LA | Solvent | T [°C] | Conv. [%]b | ee [%]c |
| 1 | L1/Cu(I) | – | CH2Cl2 | −78 | 0 | – |
| 2 | L1/Cu(I) | – | CH2Cl2 | 0 | 79d | Rac |
| 3 | L1/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 74 | 47 |
| 4 | L2/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 70 | 9 |
| 5 | L3/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 72 | 47 |
| 6 | L4/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 87 | 56 |
| 7 | L5/Cu(I) | Me3SiOTf | CH2Cl2 | −78 | 75 | 47 |
| 8 | L4/Cu(I) | Me3SiOTf | THF | −78 | 100 | Rac |
| 9 | L4/Cu(I) | Me3SiOTf | Toluene | −78 | 62 | 80 |
| 10 | L4/Cu(I) | Me3SiOTf | Ether | −78 | 91 | 88 |
| 11 | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 95 | 92 |
| 12e | L4/Cu(I) | tBuMe2SiOTf | tBuOMe | –78 | 95 | 95 |
| 13 | L4/Cu(I) | BF3·Et2O | tBuOMe | −78 | 19 | 92 |
| 14f | L4/Cu(I) | BF3·Et2O | tBuOMe | −78 | 77 | 97 |
| 15f | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 99 | 97 |
| 16g | L4/Cu(I) | Me3SiOTf | tBuOMe | −78 | 100 | 95 |
| 17 | L4/Cu(I) | Me3SiOTf | tBuOMe | 0 | 95 | 88 |
| 18 | L4/Cu(I) | Me3SiOTf | tBuOMe | −20 | 97 | 97 |
aReaction conditions: 0.1 M of 1a, 5 mol% of CuBr·SMe2, 6 mol% of L, and 2–3 equiv. of LA followed by the addition of 2–3 equiv. of EtMgBr
bConversion was determined by NMR of reaction crude
cEnantiomeric excess was determined by chiral HPLC after transforming 2a to the corresponding N,N-dimethyl amide derivative
dLess than 20% of 2a formed with many other byroducts
eThe product was obtained as a mixture of silyl ester and free carboxylic acid in the ratio of 62:38, respectively
fThe reaction was performed by first forming Li-carboxylate with nBuLi followed by addition of corresponding LA and EtMgBr
gThe reaction was performed by first forming Na-carboxylate with NaH followed by the additions of Me3SiOTf and EtMgBr
