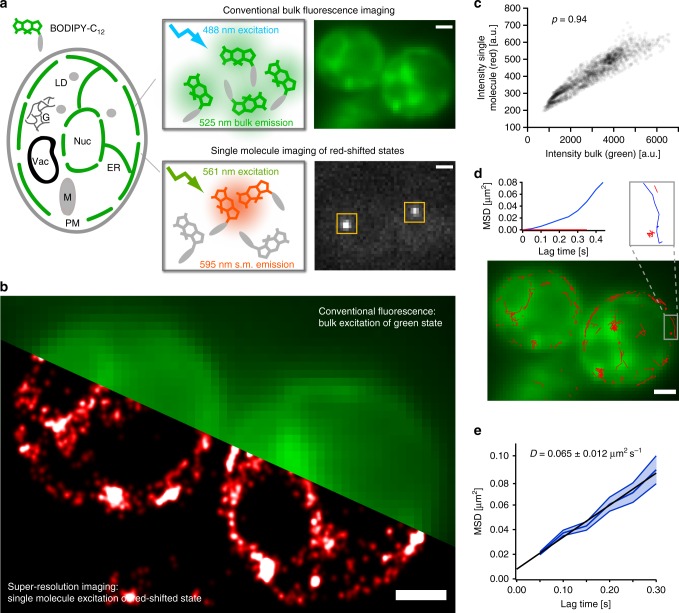
Fig. 1.
SMLM and tracking with transient red-shifted DII-states of conventional BODIPY conjugates. a BODIPY fluorophores exist as various conjugates that label specific compartments within cells. BODIPY-C12 is a fatty acid analog that localizes to the ER of living yeast cells when excited at 488 nm in the conventional fluorescence microscopy mode (green, upper). When excited at 561 nm, bright and red-shifted single-molecule fluorescence signals appear and disappear throughout the cell (white, lower). b The bright single-molecule fluorescence can be used for SMLM to obtain a ~tenfold higher resolution image of the fatty acid distribution within the cell (red). c The pixel intensities of the conventional fluoresce image of BODIPY-C12 excited at 488 nm correlate well with the single-molecule signal excited at 561 nm and averaged over 800 frames (Pearson’s ρ = 0.94, number of cells = 2, number of data points = 2128), confirming the common origin of the two signals. d The detected single-molecule signal of DII-BODIPY states lasts long enough to track fatty acid analogs (red traces). Different mobile (blue) and immobile (red) species can be discriminated. e The average mean square displacement vs. time of fatty acid analogs of 150 traces each lasting for at least 3 acquisition frames (0.15 s) results in an average diffusion coefficient of 0.065 ± 0.012 µm2 s−1 (error band: s.e.m., scale bars: 1 µm)