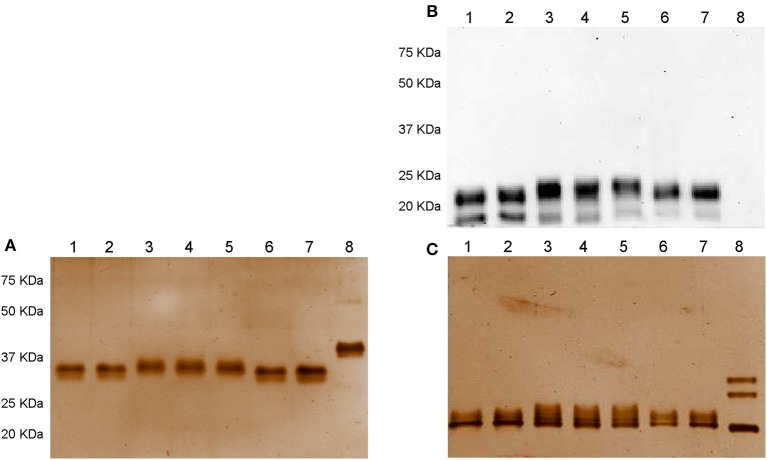
Figure 1.
Western blotting (A) and silver staining analysis (B,C) of Gonal-f® and biosimilars under non-denaturing-non reducing and denaturing-reducing conditions. Samples comprising 300 ng of each preparation, according to the quantification provided by the manufacturer, were loaded. FSH presence was detected by rabbit anti-human polyclonal primary antibody against FSHβ/FSH. Recombinant hCG was used as negative control. Samples were loaded as follows: (1) Ovaleap® batch R38915, (2) Ovaleap® batch S27266, (3) Bemfola® batch PPS30400, (4) Bemfola® batch PNS30388, (5) Bemfola® batch PNS30230, (6) Gonal-f® batch AU016646, (7) Gonal-f® batch BA045956, (8) recombinant hCG. (A) Evaluation of FSH preparations under denaturing-reducing conditions, by Western blotting, using anti-FSHβ antibody. (B) Silver staining analysis of FSH preparations under non-denaturing-non reducing conditions. (C) Analysis of FSH preparations under denaturing-reducing conditions, by silver staining.