Abstract
The diagnosis of central nervous system (CNS) infection relies upon analysis of cerebrospinal fluid (CSF). We present 4 cases of CNS infections associated with basal meningitis and hydrocephalus with normal ventricular CSF but grossly abnormal lumbar CSF. We discuss CSF ventricular–lumbar composition gradients and putative pathophysiological mechanisms and highlight clinical clues for clinicians.
Keywords: cerebrospinal fluid, cryptococcal meningitis, lumbar puncture, meningitis, tuberculous meningitis
We describe the phenomenon of ventricular/lumbar CSF discordance with normal ventricular CSF parameters in four patients with hydrocephalus due to basal meningitis and discuss possible mechanisms. In patients with hydrocephalus, a normal ventricular CSF does not exclude the possibility of underlying basal meningitis.
Analysis of cerebrospinal fluid (CSF) is a fundamental component in diagnosing central nervous system (CNS) infection. The most common methods of sampling CSF are via lumbar puncture (LP) and the sampling of ventricular CSF via insertion of an external ventricular drain (EVD) or a CSF shunt into the lateral ventricle [1–3]. In 1937, Merritt and Freemont-Smith first described the patterns of biochemical and cytological change in CNS infection. Among their seminal findings, they observed differences in composition between ventricular and lumbar CSF [1–3].
We present 4 cases of CNS infections with discrepant ventricular and lumbar CSF parameters. Published reports of such CSF discrepancies are limited [2, 4–10]. Our case series includes 2 cases of cryptococcal meningitis, for which discrepant CSF has not been previously reported.
CASE 1—CRYPTOCOCCAL MENINGITIS
A 43-year-old Vietnamese male, immunosuppressed secondary to a liver transplantation 6 years prior, presented with 12 weeks of increasing confusion, ataxia, and blurred vision. The patient was involved in a motor vehicle accident and underwent a trauma series computed tomography (CT) scan of the brain, which revealed communicating hydrocephalus, prompting the insertion of an external ventricular drain (EVD). The ventricular CSF sample taken at the time of EVD insertion was biochemically normal with normal cell counts (Table 1). The patient’s confusion and ataxia improved with insertion of the EVD. Magnetic resonance imaging (MRI) of the brain revealed a basal meningitis and communicating hydrocephalus. On day 8 of admission, an LP was performed for further diagnostic evaluation. The lumbar CSF demonstrated low glucose, high protein, lymphocytic pleocytosis (Table 1), and a positive cryptococcal antigen. The patient was commenced on therapy for cryptococcal meningitis, and a ventriculo-peritoneal (VP) shunt was inserted for management of intracranial pressure and refractory hydrocephalus. The patient made a complete recovery.
Table 1.
Cerebrospinal Fluid Biochemical, Cytological, and Microbiological Results
| Ventricular CSF | Lumbar CSF | ||
|---|---|---|---|
| Case 1 CM | Glucose (2.2–3.9 mmol/L) | 5.1 | 1.1 |
| Protein (<0.45 g/L) | 0.08 | 11.78 | |
| WCC, cells ×106/L | 0 | 86 | |
| Lymphocytes, cells ×106/L | 0 | 74 | |
| Polymorphs, cells ×106/L | 0 | 12 | |
| Erythrocytes, cells ×106/L | 132 | 132 | |
| Microbiological testing | CrAg NP, CSF culture negative | CrAg positive, CSF culture negative | |
| Case 2 CM | Glucose (2.2–3.9 mmol/L) | 3.3 | 1.0 |
| Protein (<0.45 g/L) | 0.27 | 1.03 | |
| WCC, cells ×106/L | 1 | 242 | |
| Lymphocytes, cells ×106/L | 0 | 205 | |
| Polymorphs, cells ×106/L | 1 | 37 | |
| Erythrocytes, cells ×106/L | 85 | 65 | |
| Microbiological testing | CrAg negative, no prozone effect detected, CSF culture negative | CrAg-positive CSF culture: Cryptococcus neoformans | |
| Case 3 TBM | Glucose (2.2–3.9 mmol/L) | 4.4 | 1.7 |
| Protein (<0.45 g/L) | 0.27 | 4.26 | |
| WCC, cells ×106/L | 4 | 540 | |
| Lymphocytes, cells ×106/L | 4 | 508 | |
| Polymorphs, cells ×106/L | 0 | 32 | |
| Erythrocytes, cells ×106/L | 2100 | 8 | |
| Microbiological testing | TB PCR-positive CSF culture: Mycobacterium tuberculosis | TB PCR-negative CSF, AFB c ulture negative | |
| Case 4 NCC | Glucose (2.2–3.9 mmol/L) | 4.2 | 1.1 |
| Protein (<0.45 g/L) | 0.30 | 1.01 | |
| WCC, cells ×106/L | 6 | 196 | |
| Lymphocytes, cells ×106/L | 4 | 190 | |
| Polymorphs, cells ×106/L | 2 | 6 | |
| Erythrocytes, cells ×106/L | 760 | 380 | |
| Microbiological testing | Cysticercosis serology positive | Cysticercosis serology positive |
Abbreviations: CM, cryptococcal meningitis; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; NCC, neurocysticercosis; NP, not performed; PCR, polymerase chain reaction; TB, Mycobacterium tuberculosis; TBM, tuberculous meningitis; WCC, white cell count.
CASE 2—CRYPTOCOCCAL MENINGITIS
A 40-year-old male with a history of quiescent pulmonary sarcoidosis never requiring immunosuppression presented with subacute onset of headache, vomiting, and confusion. CT of the brain demonstrated obstructive hydrocephalus, and an EVD was inserted. Ventricular CSF was biochemically normal, with normal cell counts and a negative cryptococcal antigen (Table 1). A MRI demonstrated basal meningitis and obstruction of CSF flow at the fourth ventricle due to narrowing and obstruction at the foraminae of Magendie and Luschka. A lumbar CSF obtained 4 days later demonstrated low glucose, high protein, and lymphocytic pleocytosis (Table 1). The lumbar CSF tested positive for cryptococcal antigen, and Cryptococcus neoformans was subsequently isolated. The patient commenced therapy for cryptococcal meningitis, did not need long-term CSF shunting, and made a complete recovery.
CASE 3—TUBERCULOUS MENINGITIS
A previously well 57-year-old female from Timor Leste presented with 2 weeks of fever, thoracic back pain, ataxia, and an acute decline in consciousness on the day of presentation. A CT of the brain and thoracic spine demonstrated hydrocephalus and bony destruction of the T7/8 vertebrae. An EVD was inserted, and ventricular CSF biochemistry and cell counts were normal (Table 1). MRI of the brain demonstrated nodular basal meningitis. Lumbar CSF obtained on day 4 demonstrated low glucose, high protein, and lymphocytic pleocytosis (Table 1). The ventricular CSF tested positive for Mycobacterium tuberculosis with polymerase chain reaction (PCR), and multiple ventricular CSF specimens were culture positive for M. tuberculosis. Despite the significant biochemical and cytological abnormalities, the lumbar CSF was M. tuberculosis PCR negative and culture negative after 8 weeks. The patient was initiated on TB therapy and corticosteroids but developed refractory hydrocephalus and extensive brain stem infarction. Medical care was withdrawn, and the patient died on day 38 of admission.
CASE 4—NEUROCYSTICERCOSIS
A previously well 66-year-old Laotian male presented with 2 years of progressive headache, gait disturbance, and eventual urinary incontinence. Noncontrast CT of the brain demonstrated hydrocephalus without focal abnormality. An LP demonstrated low glucose, high protein, and a lymphocytic pleocytosis (Table 1). MRI demonstrated basal meningitis, with numerous subarachnoid cystic structures throughout the prepontine basal cisterns and the lumbar cisterns, with no intraparenchymal lesions. Cysticercosis serology on CSF and serum was strongly positive, and a diagnosis of racemose neurocysticercosis was made. The patient was transferred to our hospital for neurosurgical management. A VP shunt was inserted for obstructive hydrocephalus. Ventricular CSF results at the time of insertion demonstrated normal biochemistry, with CSF cell counts within the upper limits of normal after adjusting for an elevated CSF erythrocyte count (Table 1). Cysticercosis serology on the ventricular CSF was also positive. The patient received a prolonged course of combination antiparasitic therapy and tapering steroids and was symptomatically well at last follow-up 15 months after diagnosis.
DISCUSSION
These 4 cases highlight the phenomenon of discordant CSF parameters from different CSF compartments. Remarkably, the ventricular CSF results fell within normal limits, highlighting the potential for diagnostic error and delay.
Table 2 summarizes the normal biochemical and cytological gradients that are thought to exist in the CSF and general changes in pathological states. The majority of CSF is formed by the choroid plexus within the cerebral ventricles. CSF flows through the interventricular foramina into the subarachnoid space, with the majority of CSF flowing over the cerebral hemispheres to be reabsorbed into the sagittal venous sinus (Figure 1). Only a small proportion of CSF circulates down into the lumbar cistern.
Table 2.
Table Comparing Differences Between Ventricular CSF and Lumbar CSF in States of Health and Disease
| Parameter | Ventricular CSF | Lumbar CSF | Normal Physiology | Changes in Pathology |
|---|---|---|---|---|
| Protein | Lower | Higher | Protein is progressively added through normal CSF circulation. 80% is derived from serum. | Increased ventricular–lumbar gradient in most disease processes; the magnitude of difference varies. |
| Normal ventricular:lumbar ratio of 0.25:0.60 in health. | ||||
| Glucose | Similar | Similar | Similar concentrations in healthy individuals. | Ventricular glucose significantly higher than lumbar glucose in most CNS infections. |
| Strong correlation to serum levels of glucose. | Multifactorial mechanisms and varies in disease states. Proposed mechanisms include increased microbial consumption, increased brain metabolic activity, consumption by WBC or malignant cells, and impaired function of the BBB & choroidal plexus. | |||
| WBCs | Lower | Higher | WBC gradient exists in health based on limited data. Mechanisms not clear. | Increased gradient in most disease processes, but degree depends on cause and location of abnormality in the CNS. |
| Reversal of gradient can occur with predominant ventricular pathology. | ||||
| Lactate | Similar | Similar | A mild cranio-caudal gradient exists, but much more uniform distribution through all compartments. | Elevated levels from CNS anaerobic glycolysis due to any condition that results in cerebral hypoperfusion, or produced by increased concentration of WBCs. |
| Produced within the CSF and generally independent of serum levels. | Retains more uniform distribution even in states of disease. |
Abbreviations: BBB, blood brain barrier; CNS, central nervous system; CSF, cerebrospinal fluid; WBC, white blood cell.
Figure 1.
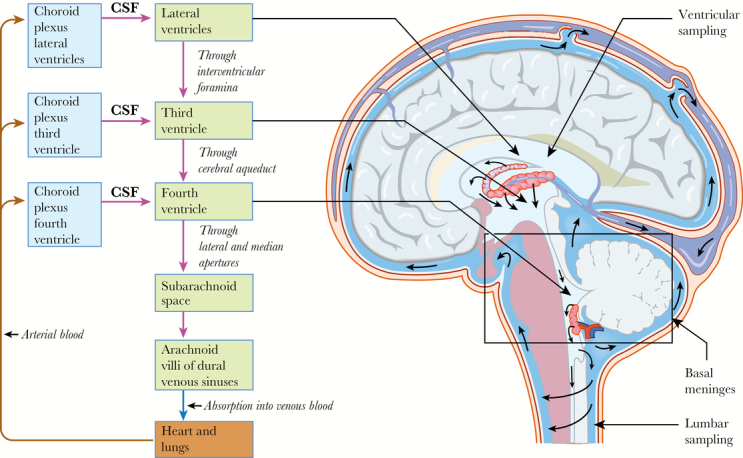
CSF is produced in the choroid plexus of the lateral, third, and fourth ventricles. The CSF flows unidirectionally from the lateral ventricle to the third ventricle, to the fourth ventricle, and then to the subarachnoid space in the region of the basal meninges. Ventricular sampling of the CSF is from the lateral ventricles, and lumbar sampling is from the lumbar cisterns in communication with the subarachnoid space. Only a minority of CSF flows into the lumbar cisterns, with the rest reabsorbed by the arachnoid villi of the dural venous sinuses. Abbreviation: CSF, cerebrospinal fluid.
The magnitude of differences between ventricular and lumbar CSF can vary, especially in pathological states [1–4]. Even in advanced CNS infection, the ventricular CSF can be biochemically normal with normal cell counts, while being grossly abnormal in the lumbar compartment [1, 2, 4–12]. This phenomenon is most strikingly demonstrated in patients who have developed hydrocephalus, which is the usual indication for ventricular drain insertion [4–7, 11, 12]. An underappreciation of these nuances is an important source of diagnostic error, which can lead to delay in or misdiagnosis of life-threatening CNS infection [4–7].
Three of the 4 cases were associated with a degree of mechanical obstruction to flow as a contributing factor to the development of hydrocephalus. None of these cases involved cases of iatrogenic meningitis (following CSF drainage), which is the main patient population in which ventricular and lumbar CSF comparisons have been performed [2, 4, 12]. Our cases had minimal ventriculitis, with predominantly basal meningitis. Case 4 with neurocysticercosis had subarachnoid cysts located around the basal meninges, with minimal intraventricular and no intraparenchymal cysts.
All etiological diagnoses in our case series are classical causes of basal meningitis. The basal meninges are notable for their intimate location within the median and lateral apertures, through which CSF exits the fourth ventricle, and for a predisposition to complications of hydrocephalus. Basal meningitis syndromes, such as tuberculous meningitis, cryptococcal meningitis, and neurocysticercosis, are shown here to be potentially associated with discrepant ventricular and lumbar CSF, especially when there is associated hydrocephalus.
Microbiological testing can also be discordant between ventricular and lumbar CSF. Case 2 demonstrated a negative cryptococcal antigen on ventricular CSF compared with lumbar CSF in the context of ventricular obstruction. In Case 3, interestingly, the ventricular CSF was TB PCR positive and repeatedly culture positive despite normal biochemistry and cell counts, whereas the lumbar CSF was TB PCR negative and culture negative but had abnormal biochemistry and cell counts. The total volume of lumbar CSF tested was 4 mL for Case 3, and may have reduced the diagnostic yield. Studies in extensive subarachnoid neurocystercerosis highlight similar problems of compartmentalization affecting testing [7, 8].
Case 1 highlights that mechanical obstruction to CSF flow is not always present. Even in nonobstructive hydrocephalus, discordance in CSF results can still exist. It is likely that 3 main factors contribute to the large CSF concentration gradients in basal meningitis syndromes. First, the normal direction of CSF flow, where sampling “downstream” of intense basal inflammation results in more significant abnormalities compared with the CSF “upstream” in the ventricles. Second, the pathophysiological addition or subtraction of solute (as described in Table 2) closely relates to sampling downstream to the direction of the flow. Third is gravity, where solutes collect in higher concentrations in dependent areas of CSF such as the lumbar cistern. Similarly, although antigens and nucleic acid products may be soluble within CSF, the direction of CSF flow may create a “concentration gradient” with resultant differential yield on diagnostic assays depending on location of sampling.
CONCLUSIONS
We describe 4 instructive cases of CSF discordance between ventricular and lumbar CSF parameters in patients presenting with hydrocephalus associated with basal meningitis syndromes. A normal ventricular CSF does not rule out a CNS infection. In addition to clinical and radiological clues, host factors such as country of origin and immunocompromised states should heighten awareness of basal meningitis. In patients with hydrocephalus, where there is diagnostic uncertainty or clinical suspicion of basal meningitis syndromes, an LP should be performed. Our experience highlights to clinicians an important source of potential diagnostic error in this patient group.
Acknowledgments
Potential conflicts of interest. Medical illustrations were created and formatted using an independent medical illustrator. No declarations or conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. S.K. performed the literature review, writing of the manuscript, and provision of patient care. T.M. performed data collection and writing of the manuscript. C.H. assisted with manuscript review and provision of patient care. O.X. assisted with manuscript review and provision of patient care. N.H. assisted with manuscript review and provision of patient care. S.M. assisted with manuscript review and provision of patient care. I.J. assisted with manuscript review and provision of patient care. S.T. assisted with manuscript review, coordination of the manuscript, and provision of patient care. A.S. assisted with manuscript review, coordination of the manuscript, and provision of patient care.
References
- 1. Merritt HH, Fremont-Smith F.. The Cerebrospinal Fluid. Philadelphia: WB Saunders; 1937. [Google Scholar]
- 2. Sommer JB, Gaul C, Heckmann J, et al. . Does lumbar cerebrospinal fluid reflect ventricular cerebrospinal fluid? A prospective study in patients with external ventricular drainage. Eur Neurol 2002; 47:224–32. [DOI] [PubMed] [Google Scholar]
- 3. Watson MA, Scott MG. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin Chem 1995; 41:343–60. [PubMed] [Google Scholar]
- 4. Gerber J, Tumani H, Kolenda H, Nau R. Lumbar and ventricular CSF protein, leukocytes, and lactate in suspected bacterial CNS infections. Neurology 1998; 51:1710–4. [DOI] [PubMed] [Google Scholar]
- 5. Alfayate-Miguélez S, Martínez-Lage-Azorín L, Marín-Vives L, et al. . Normal ventricular-CSF may confound the diagnosis of tuberculous meningitis hydrocephalus. Neurocirugia (Astur) 2011; 22:157–61. [DOI] [PubMed] [Google Scholar]
- 6. Said M, Uppal P, Bye A, Palasanthiran P. Unusual case of tuberculous meningitis with discordant ventricular and lumbar cerebrospinal fluid; lessons in the era of world-wide migration. J Paediatr Child Health 2018; 54:93–5. [DOI] [PubMed] [Google Scholar]
- 7. Rubalcava MA, Sotelo J. Differences between ventricular and lumbar cerebrospinal fluid in hydrocephalus secondary to cysticercosis. Neurosurgery 1995; 37:668–71; discussion 671–2. [DOI] [PubMed] [Google Scholar]
- 8. Torres-Corzo JG, Tapia-Pérez JH, Sánchez-Aguilar M, et al. . Comparison of cerebrospinal fluid obtained by ventricular endoscopy and by lumbar puncture in patients with hydrocephalus secondary to neurocysticercosis. Surg Neurol 2009; 71:376–9. [DOI] [PubMed] [Google Scholar]
- 9. Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 2011; 8:3:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chow E, Troy SB. The differential diagnosis of hypoglycorrhachia in adult patients. Am J Med Sci 2014; 348:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol 2001; 3:42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naija W, Matéo J, Raskine L, et al. . Case report: greater meningeal inflammation in lumbar than in ventricular region in human bacterial meningitis. Crit Care 2004; 8:R491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


