Summary
Neuroimaging techniques applied to a variety of organisms from zebrafish, to rodents to humans can offer valuable insights into neuronal network properties and their dysfunction in epilepsy. A wide range of imaging methods used to monitor neuronal circuits and networks during evoked seizures in animal models and advances in fMRI applied to epilepsy patients were discussed during the XIV Workshop on Neurobiology of Epilepsy (XIV WONOEP) organised in 2017 by the Neurobiology Commission of the International League Against Epilepsy (ILAE). We review the growing number of technological approaches developed, as well as the current state of knowledge gained from studies applying these advanced imaging approaches to epilepsy research.
Keywords: fMRI, Calcium Imaging, Neuroimaging, Graph theory, Epileptic networks
1. Introduction
Epilepsy may be broadly defined by a state of enduring predisposition to seizures, which arise when the balance between excitation and inhibition is disrupted in the context of abnormal synchronization. Epileptogenesis can be examined at different “levels” of the nervous system: first at the level of the molecular building blocks including genes, proteins, ions and membranes1, then cells and circuits/synapses, and finally large-scale neuronal networks. Both due to the complexity of molecular disease mechanisms and the architecture of neuronal networks, application of systems biology approaches based on network neuroscience has found its entrance in epilepsy research. At its simplest, a network is a collection of items (called nodes) that possess pairwise relationships (called edges). The brain as a whole can be considered a hierarchically organised network, partitioned into mutually-interconnected units responsible for information processing spanning from local circuits to broad functional areas. A network perspective has a particular relevance in epilepsy, since structures within an epileptogenic network are thought to be involved in the generation and expression of seizures, and to the maintenance of the disorder2.
For this review, we will focus on the network concept in epilepsy from an imaging perspective. Compelling evidence from preclinical models, experimental paradigms, and humans indicates that specific cortical and subcortical networks play a fundamental role in the genesis and expression of seizures. In the last two decades, converging evidence from neuroimaging literature has shown distributed anomalies in the neocortex and the white matter in epilepsy syndromes associated with structural abnormalities, challenging the conventional model of focal epilepsy (reviewed in3). The hypothesis that focal epilepsy may be more adequately described as a system-level disorder is now a bourgeoning research area fuelled by advances in connectomics4. Recent technological advances in imaging of neuronal activity in animal models, and progress in structural and functional MRI in human studies have made it possible to examine regional networks involved in seizure onset and propagation, and to identify those displaying abnormal functional connectivity during inter-ictal periods. In parallel, ongoing efforts aim at using neuroimaging to predict epileptogenesis as well as ictogenesis5. In this article, we will provide an overview of high-end neuroimaging techniques used to study neuronal activity in vivo in animal models of seizures and epilepsy, and we will show how these studies are improving our understanding of local and distributed networks in ictogenesis. PET imaging studies have been thoroughly reviewed elsewhere5. We will also discuss the current state of knowledge gained by advancing MRI-imaging approaches to map whole-brain and epileptic networks in patients.
2. Animal studies
Traditionally, electrophysiological recordings have been used to evaluate seizure activity in vivo, and benefit from high sampling rates. Imaging approaches can however offer substantial spatial advances over electrophysiology. Even when high density surface arrays are employed, there can be difficulties in accurately localising neuronal activity due to low spatial sampling and volume conduction6. Multi-electrode extracellular probes can record simultaneously from hundreds of neurons7, but their readout is spatially restricted and limited to active neurons. Determining cell type firing is difficult, particularly during a seizure when distortions of action potential waveform prevent spike sorting8. It has been known for many years that intrinsic optical imaging of haemodynamic signals can be used to monitor epileptiform activity and seizure propagation in vivo9; however, this approach has limitations. Renewed interest in high resolution in vivo imaging was sparked only recently, due to the rapid and continuous advancement of fluorescent microscopy, development of novel fluorophores and importantly data analysis algorithms.
2.1. Novel optical imaging methods to monitor neuronal activity
Recent advances in microscopy, coupled with the ability to express calcium and voltage sensitive indicators in subclasses of neurons in zebrafish and rodents, using either viral vectors or transgenic approaches, have resulted in the ability to image neuronal activity in exquisite detail. These methods have been used to determine the roles of different neuronal subclasses and local circuits during behavioural tasks in awake animals and have recently been applied to epilepsy research characterising epileptic networks at multiple scales, from neuronal microcircuits to brain wide networks. These approaches rely on fluorescent reporters of neuronal activity. Bolus loading of traditional organic dyes capable of reporting changes in intracellular calcium or membrane voltage have proved very useful. However, cell specificity can be achieved using genetically encoded fluorescent reporters10. Genetically encoded indicators of calcium (GECI) or voltage (GEVI) have become the approach of choice, owing to the ever increasing improvements in transgenic technologies11, viral vectors12 and fluorescent reporters, including those with red-shifted excitation and emission spectra, which allow deeper in vivo imaging and reduced phototoxicity13. Despite having superior temporal resolution, voltage sensitive indicators suffer from low signal to noise ratio (SNR) and are not optimal for detecting neuronal inhibition or distinguishing between subthreshold depolarisations and action potentials in vivo14. In contrast, calcium indicators have a high SNR and a broad dynamic range15 and are more commonly used in epilepsy research, despite their slower temporal properties. When a neuron fires an action potential calcium enters the cells and GCaMP fluorescence transiently increases. Therefore, an increase in fluorescence can be used to identify neurons firing during a seizure. Modified and enhanced members of the GCaMP family of indicators16 are usually expressed in specific classes of neurons using either viral-vector mediated approaches or through creation of transgenic organisms. Care should be taken to ensure that expression levels of indicators are optimised to avoid off-target effects17.
To understand how local and distributed networks are involved in epilepsy, different imaging approaches can be employed. To detect changes in intrinsic excitability, local imbalance in excitation and inhibition and the initiation of seizures from a discrete focus, imaging of densely packed neuronal populations will be beneficial, ideally at single cell resolution and with indicators expressed in defined neuronal populations from the seizure onset zone. However, the ictal focus does not operate in isolation and is connected to other areas of the brain via short and long-range projections. Alternative mesoscopic imaging techniques are instead required to record propagation pathways and areas of the brain recruited for seizure “pace-making”, or to detect inter-ictal abnormalities between functionally connected but distant areas of the brain. Mesoscopic imaging refers to a spatial scale between microscopic and macroscopic. This approach does not permit single neuron imaging but is a powerful approach to determine areas of brain with enhanced neuronal firing rates. Neuronal activity can also be visualized in fixed tissue utilizing genetically modified mice that express fluorescent proteins under the control of early immediate genes, such as c-Fos and c-Jun. While, early immediate gene mRNA and protein expression have been used to map seizures, the transient nature of expression and low SNR limit their use. In recent years, genetically modified mice have been developed that express fluorescent proteins such as tdTomato or GFP in neurons under the control of early immediate gene promoters. These promoters can be temporally controlled by drug binding sites for tetracycline or tamoxifen18. Tissue clarifying techniques further facilitate identification of neurons expressing fluorescent proteins. Brain tissue of various thickness can be clarified using active or passive clarification techniques19; 20, and detailed 3D images using confocal or two photon imaging can be obtained. In addition, these whole-brain microscopy approaches can also be used in co-registration with in vivo mesoscopic imaging modalities21.
The imaging approaches most commonly applied to epilepsy studies and discussed at the WONOEP meeting included 2-Photon, wide-field and light sheet fluorescent microscopy, the use of miniscopes for chronic recordings in freely moving animals, and analysis of blood-oxygen-level dependent (BOLD) signal during fMRI. A brief description highlighting the differences and particular advantages each of these techniques is provided in Supplementary Box 1. A detailed review of the growing number of microscopy techniques available for in vivo imaging of neuronal activity has recently been published22.
2.2. Preclinical models of epilepsy
As zebrafish and mice are genetically amenable, they have become powerful model organisms for analysing genetic diseases. It is possible to derive transgenic lines that harbour the same mutations in genes found in human forms of genetic epilepsy. For instance, zebrafish and mouse models of Dravet syndrome have been developed23; 24. These models could be used for detecting development of network abnormalities in genetic forms of epilepsy and may aid our understanding of the functional consequences of pathophysiological activity patterns from a cellular level to large scale cortical networks. In models of acquired chronic epilepsy, there will be both pro-epileptic and compensatory changes of the network and neuronal firing patterns. A major complication that hampers imaging of spontaneous seizures is their low frequency. For this reason, the majority of neuroimaging studies have focused on acute pharmacologically or electrically induced seizures or have monitored inter-ictal abnormalities in chronic epilepsy models.
2.3. Insights obtained from in vivo imaging studies
Calcium imaging of seizure activity from head-fixed rodents.
Combining 2-photon calcium imaging with a chronic model of temporal lobe epilepsy, Muldoon and colleagues25 examined the microcircuits that participate in inter-ictal spikes in awake animals. Epileptiform activity was recorded through electrophysiological recordings in one hippocampus and via calcium imaging in the contralateral hippocampus. Although there was variability in the cellular dynamics of inter-ictal spikes, GABAergic neurons are thought to be preferentially recruited during spontaneous inter-ictal activity in hippocampal CA1 region25. Notably, few studies have reported positive associations between inter-ictal spiking, aberrant hippocampal interneuron firing and cognitive impairment, suggesting a plausible link between GABAergic activation and cognition26.
Several studies have examined the role of inhibitory restraint26 during seizure activity using a combination of 2-Photon calcium imaging and local field potential recordings. In response to chemoconvulsant-evoked seizures, neuronal populations within and across cortical layers are recruited in a reliable manner propagating with similar spatial directions. Temporal dynamics were however variable across seizures and relied on GABAergic input from the inhibitory surround27. Using a similar methodological approach, Liou and colleagues demonstrated that inhibition not only plays an important role in containing seizure invasion close to adjacent cortex, but also protects areas distant from the seizure focus. Acute focal breakdown of distant inhibition allows the development of a secondary focus, with seizures in this area triggered by input from the original focus28.
Dysfunctional astrocytes have been proposed to play an important role in epilepsy29, and the tools required to image their calcium signalling in vivo are available. Two recent studies employing either 2-Photon or wide-field florescent microscopy have determined that, although seizures induce a large calcium wave through the astrocytic syncytium, the latter occurs after seizure onset, is spatio-temporally uncoupled from faster neuronal activity and terminates before neuronal activity30; 31. These investigations suggest that the propagating glial calcium wave is not required for ictal initiation and propagation, as blockade of glial activity had no impact on seizure spatiotemporal dynamics30. These experiments were conducted in ‘healthy’, non-epileptic brains and seizures were induced using chemoconvulsants, therefore it cannot be excluded that astrocyte dysfunction in established epilepsy may occur, and that astrocytes may have a role in ictogenesis.
The spatiotemporal evolution of epileptiform activity in the awake cortex and its relationship to the underlying functional connectivity was recently investigated using wide-field imaging32. The functional connectivity within and across visual areas can be easily mapped, and the contribution of both local and long-range connections to the propagation of epileptiform discharges can be detected. This study demonstrates that both inter-ictal spikes and seizures evoked in the primary visual cortex start as standing waves in the V1 focus and in homotopic locations in higher visual areas. Seizures then propagate as a traveling wave across adjacent cortex, and jump to invade homotopic distal regions32.
Calcium imaging of seizure activity from freely moving rodents.
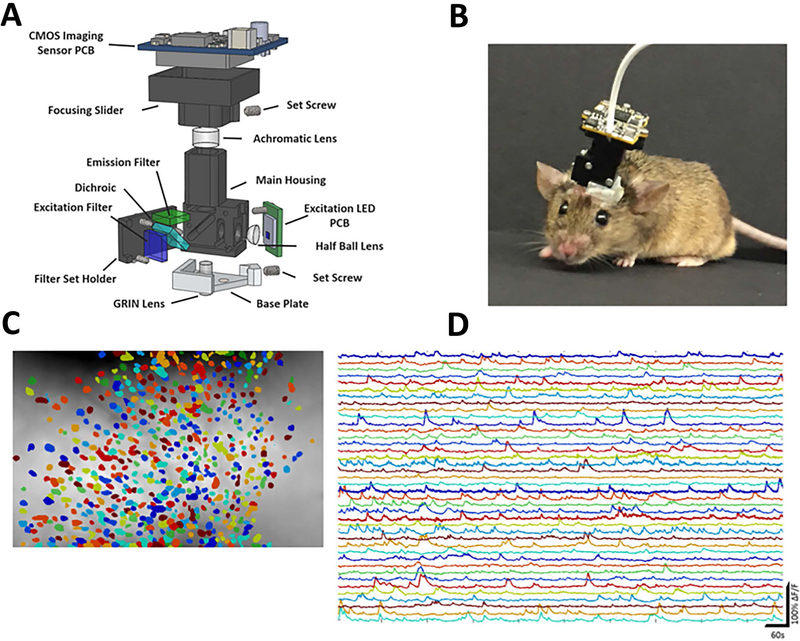
New miniaturized microscopes33 combined with genetically encoded calcium indicators now allow recordings of activity from hundreds of neurons simultaneously in freely behaving animals. The use of miniaturized microscopes has several distinct advantages over electrophysiological techniques. Most importantly, unlike electrophysiological recordings where the same neuron can only be followed for 1–2 days, miniaturized microscope calcium imaging can follow the activity of the same sets of neurons for weeks (Figure 1). This allows the investigator to determine how each neuron changes its firing patterns after learning or a disease related insult. Second, the number of neurons investigated simultaneously is nearly an order of magnitude greater with calcium imaging, especially in mice which cannot carry a large number of electrode drives because of weight limitations. Last, the use of imaging allows expression of GCAMP6 in specific cell types or specific projection neurons34. While miniaturized microscopes from commercial sources are extremely costly, new open-source versions created by Daniel Aharoni in the Golshani, Silva, and Khakh labs are nearly 50 times less expensive and have allowed over 500 labs to quickly and easily build their own miniaturized microscopes. The Golshani group is using these microscopes to understand the mechanisms underlying poor spatial memory in temporal lobe epilepsy. By recording place related activity in thousands of CA1 neurons over a week, results show dramatic reductions in the number, precision, and stability of place fields35.
Figure Legend 1:
Calcium imaging of neuronal activity in freely moving mice.
A. Schematic demonstrating all the components of the open-source miniaturized microscope. B. Photograph of mouse walking with a miniaturized microscope imaging the hippocampus. C. Schematic demonstrating a subset of the neurons imaged from hippocampal CA1 in freely behaving mice. D. Calcium traces from neurons demonstrated in C.
BOLD signals during fMRI.
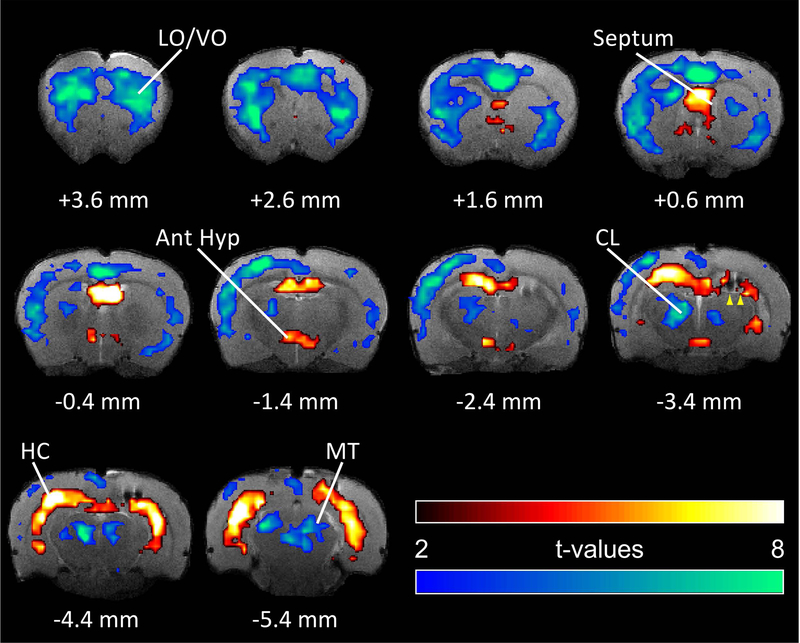
BOLD fMRI is widely used in human studies of focal and generalized epilepsy36; 37. Investigations that employ fMRI in epilepsy animal models therefore have direct translational value. However, it is important to be cautious when interpreting BOLD fMRI because signals are only indirectly related to neuronal activity, sometimes resulting in paradoxical effects, particularly in subcortical structures or during epileptiform activity38. These concerns can be overcome through direct neuronal recordings to validate fMRI findings39. The combination of BOLD fMRI mapping followed by direct neuronal recordings has led to crucial new insights into network mechanisms in epilepsy. For example, impaired cortical function and consciousness in hippocampal seizures is associated with fMRI increases in subcortical regions including the lateral septum and anterior hypothalamus40 (Figure 2). Follow-up direct electrical recordings confirmed increased neuronal firing in inhibitory regions such as the lateral septum, which can depress subcortical arousal leading to impaired consciousness in focal seizures40; 41. Similar subcortical inhibitory mechanisms may play a role in depressed cardiorespiratory function and sudden unexpected death in epilepsy (SUDEP). The network understanding gained through fMRI has also guided potential therapeutic interventions for restoring consciousness during focal seizures through electrical or optogenetic stimulation of subcortical arousal systems42; 43. BOLD fMRI with electrophysiological verification and diffusion-tensor imaging (DTI) have also yielded crucial insights into mechanisms of seizure generation38; 44; 45 as well as developmental epileptogenesis and epilepsy prevention46–49.
Figure Legend 2:
BOLD fMRI changes during focal limbic seizures in a rat model.
T-map of ictal changes during focal seizures induced by brief 2s hippocampal stimulation (10 animals, 34 seizures, mean seizure duration ± SEM 70.72 ± 4.01 s). Widespread cortical decreases are accompanied by mixed subcortical increases and decreases. Increases are in known areas of seizure propagation such as the hippocampus (HC) and lateral septum as well as in sleep-promoting regions such as the anterior hypothalamus (Ant Hyp). Decreases are seen in the cortex, most prominently in lateral and ventral orbital frontal cortex (LO/VO) and in medial regions including cingulate and retrosplenial cortex. Decreases are also seen in arousal promoting regions such as the thalamic intralaminar nuclei including centrolateral nucleus (CL), as well as in the midbrain tegmentum (MT). Arrowheads at AP −3.4 mm signify hippocampal electrode artifact. Warm colors represent fMRI increases, and cool colors decreases. Reproduced with permission from reference41.
3. Contribution of in vivo imaging to understand the network properties of epilepsy in humans
By offering several sensitive and versatile whole-brain tissue markers, MRI has improved our ability to non-invasively map epileptogenic lesions and has revolutionized the management of patients with pharmacoresistant epilepsy, shifting the field from prevailing electroclinical correlation to a multidisciplinary approach. Given the relevance for surgical target identification, initial neuroimaging studies focused on the detection of brain lesions; indeed, the resection of a lesion detected on MRI is currently the best predictor of post-surgical seizure freedom50; 51. Over the last two decades, an increasing number of studies have also shown structural changes affecting distributed regions across the neocortex and the axonal bundles linking them, suggesting widespread abnormalities of brain organization. While the majority of studies have so far focused on temporal lobe epilepsy, initial evidence of widespread structural and functional reconfigurations is also emerging for epilepsies secondary to cortical malformations52. Moreover, abnormalities outside the lesional boundaries have been shown to negatively impact seizure outcome after surgery, which is still suboptimal in up to 40% of patients despite rigorous selection53; 54. These findings have prompted a major conceptual shift from the conventional interpretation of focal epilepsies and emphasize the importance of a network approach to adequately capture the neurobiology of this disorder. In this section, we will discuss findings related to whole-brain network alterations and those associated with epileptic spikes.
3.1. Network modelling using structural and functional MRI
Methodological advances in non-invasive neuroimaging have led to map structural and functional networks in vivo. While structural networks can be inferred from diffusion MRI tractography and inter-regional covariance patterns of structural measures such as cortical thickness, functional connectivity is generally computed based on statistical dependencies of neurophysiological time-series, measured through functional MRI techniques. In addition, network science offers increasingly sophisticated analytical methods to parametrize topology and organizational properties of large-scale networks (reviewed in55).
3.2. Network studies in drug-resistant epilepsy - insights from temporal lobe epilepsy
Temporal lobe epilepsy is associated with widespread abnormalities affecting temporo-limbic circuits as well as several large-scale networks. Morphometric correlation analyses revealed decreased structural coordination between mesiotemporal regions and numerous neocortical areas56. Covariance of atrophy between the thalamus, mesiotemporal lobe and multiple frontal and temporal cortices points to a prominent involvement of this subcortical region in the pathologic network57. Considering the underlying white matter, severe abnormalities in multiple diffusion markers point to a reconfiguration of the architecture of several temporo-limbic tracts, with changes displaying a progressive reversal as a function of the anatomical distance from the epileptogenic focus58. Diffusion derangements also encompass specific connections arising from the thalamus, such as ipsilateral anterior thalamic radiation, and tracts linking ipsilateral thalamus with the pre-central gyrus59. Diffusion changes display a progressive reversal as a function of the anatomical distance from the epileptogenic focus58.
3.3. Graph theory - a formal framework to model network topology
Conventional analysis approaches, mainly based on between-group comparisons, can capture disease-related regional and connectional alterations. However, they are not tailored to address topological aspects of whole-brain interactions. Graph theory, a framework for the mathematical representation and analysis of complex systems, has attracted considerable attention as it provides a powerful formalism to quantitatively describe the organizational patterns of brain networks. The global topology of healthy brain networks is characteristic of a small-world, an architecture that enables functional specialization and integration at relatively low wiring costs. Small-world networks are defined by tightly inter-connected nodes, which are themselves linked to other nodes through few inter-connector links. Modularity, i.e., network decomposability into smaller communities, offers adaptability and robustness to changing environmental conditions. Modularity is undermined by disease processes60. Besides the characterization of global and modular properties of large-scale networks, graph-theoretical techniques allow the localization of core regions, so-called hubs, through centrality-based metrics61. Hub regions are more densely interconnected than would be expected from a rich-club subnetwork. Interestingly, the latter encompasses mostly long-range connections, indicating its role as a backbone for cross-module connectivity62 and functional diversity63.
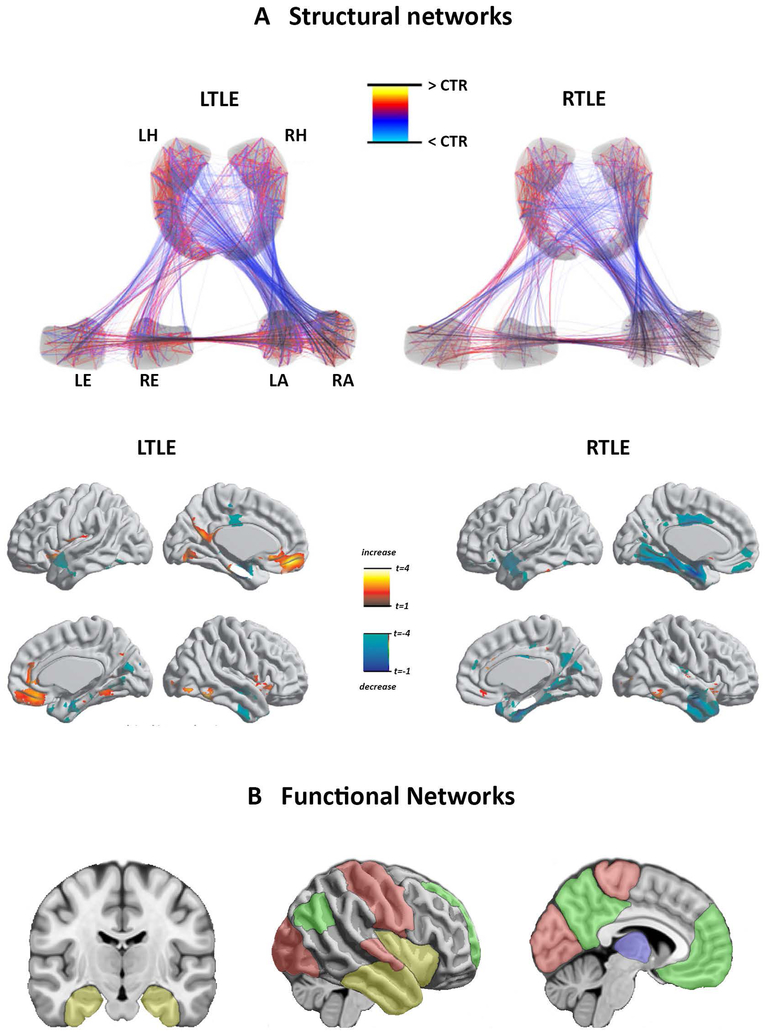
In temporal lobe epilepsy, nearly all studies have focused on patients with a unilateral seizure focus. Graph-theoretical studies based on structural MRI have shown profound rearrangements within mesiotemporal lobe subnetworks54; 64; 65, with a shift towards a more regularized topology64 (Figure 3A). Analyses of functional data also showed deranged limbic nodal topology66 and changes suggestive of compensatory reorganisation of the contralateral networks66. The severity of topological anomalies within and outside the temporal lobe positively scale with the degree of hippocampal sclerosis, indicating a major role of the epileptogenic lesion in the remodelling of whole-brain networks67,64; 68. Regularization of whole-brain network topology as well as pronounced shifts in the distribution of hubs and modularity were collectively reported across modalities, including structural MRI, diffusion MRI, and EEG-derived networks69. Graph-theoretical studies also indicated reduced coupling between structural and functional networks, which may be partially modulated by disease duration70.
Figure Legend 3:
Network abnormalities in temporal lobe epilepsy.
A. The upper panel of section shows differences in structural covariance of mesiotemporal subnetworks between patients with a left (LTLE) and right temporal lobe epilepsy (RTLE) and controls (CTR), pointing to striking reconfigurations of mesiotemporal connectivity (adapted with permission from reference64. The lower panel displays abnormalities of structural connectivity of the ipsilateral entorhinal cortex in LTLE and RTLE compared with healthy controls, suggesting a reorganization of temporo-limbic and default-mode networks (reference56 adapted with permission). B. Maps show cortico-subcortical regions exhibiting aberrant functional connectivity in TLE, mostly belonging to temporo-limbic, default-mode, sensory-motor and thalamo-cortical networks [adapted from reference55 under the terms of Creative Commons Attribution License (CC BY)]. Abbreviations: LA/RA = left/right amygdala; LE/RE = left/right enthorinal cortex; LH/RH = left/right hippocampus.
With respect to functional connectivity measures, resting-state fMRI (rs-fMRI) studies found impaired connectivity of mesiotemporal structures, mostly involving links between anterior and posterior hippocampus, and between anterior hippocampus and entorhinal cortex, ipsilaterally71. Reduced functional connectivity was additionally detected between ipsi- and contralateral hippocampus, insula, and between ipsilateral mesiotemporal structures and bilateral lateral temporal neocortices66; 72. Altered functional integration has been found between mesiotemporal and subcortical structures, including the thalamus73–77, and may co-exist with enhanced connectivity in contralateral mesiotemporal networks71. At a whole-brain level, bilaterally impaired functional connectivity has been consistently detected for areas pertaining to the default mode network (DMN), which is traditionally composed of mesiotemporal lobes, mesial prefrontal, lateral and midline parietal areas67; 73; 76. Connectional derangements in temporal lobe epilepsy have also been documented for sensory-motor, attentional, episodic memory, working memory, and language networks, supporting the pervasive nature of the disease, affecting multiple systems (Figure 3B).
Although neuroimaging-derived structural and functional abnormalities show considerable overlap in temporal lobe epilepsy, relatively few studies have directly addressed cross-domain relationships. Decreased network integration of the hippocampus could be partially explained by estimates of its grey matter density76. Recent data suggest that the magnitude of hippocampal structural damage may relate to the extent of its functional disconnection from the DMN68. Moreover, disrupted functional connectivity between mesiotemporal structures and neocortical targets was associated with altered diffusion parameters of the interconnecting white matter tracts76. In a recent study, abnormal function of midline and lateral default mode areas were shown to be mediated by microstructural abnormalities of the temporo-limbic superficial white matter78. In addition to the effects of structural damage and possibly seizure activity itself, emerging data shows evidence for effects of anti-seizure drugs on cognitive networks79; 80. Prospective studies in patients with new-onset epilepsy may help disentangle medication related effects from those related to seizures.
3.4. Combined analysis of EEG and functional MRI to analyse epileptic networks
EEG-functional MRI (EEG-fMRI) is a non-invasive tool that combines electrical and hemodynamic information. The regions of hemodynamic changes are presumed to be involved in the abnormal neuronal activity at the time of epileptic discharges37; 81–83. Regardless of the aetiology and type of epilepsy syndrome, inter-ictal scalp EEG-fMRI analyses in patients with focal epilepsy often reveal distributed patterns of BOLD activation, usually with the maximum in the presumed epileptogenic zone37, and secondary clusters in remote ipsi- and contralateral cortices, as well as subcortical regions, interpreted as epileptic networks84–86; conversely, deactivation tends to occur in the DMN.
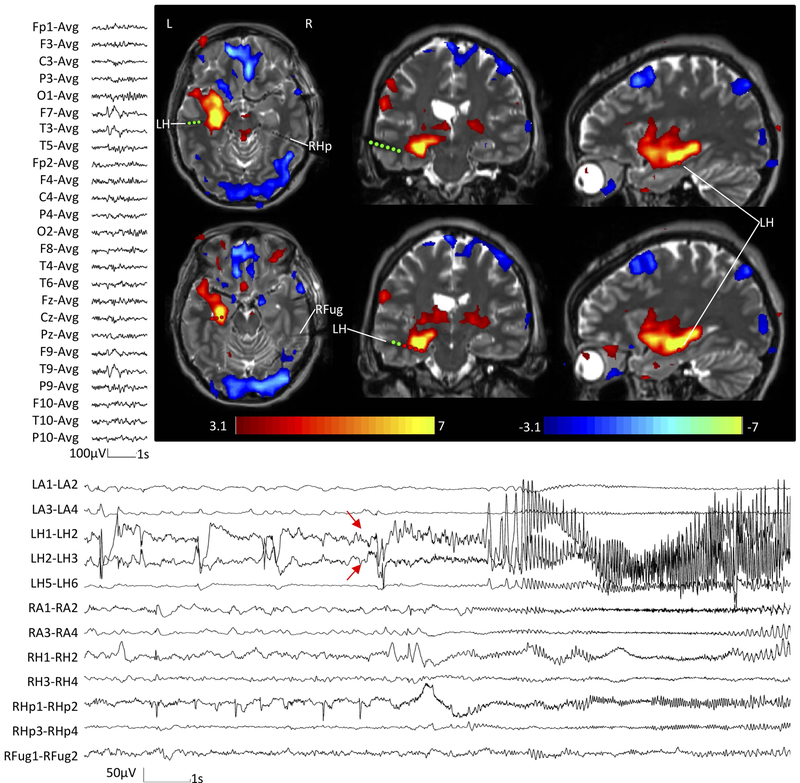
In temporal lobe epilepsy, EEG-fMRI studies have indicated that activations correlated with temporal lobe interictal discharges encompass a widespread ipsilateral network, most frequently extending to an ensemble of limbic and subcortical structures84 (Figure 4). The widespread nature of epileptogenic networks implies increased functional connectivity between the epileptogenic region and remote brain areas, possibly with patient-specific connectional profiles87. Functional abnormalities have even been shown in regions unaffected by epileptic discharges88, suggestive of a widespread pathological process that alters whole-brain intrinsic functional network architecture. Despite its limitation to only patients with subclinical seizures or seizures with very little movements, a few studies described the epileptic network associated with ictal activity during EEG-fMRI89; 90. Overall, results suggest that seizure onset is limited to a single region, while seizure propagation involve a complex network.
Figure Legend 4:
EEG-fMRI findings in temporal lobe epilepsy.
In this example, EEG-fMRI hemodynamic responses (top right) correlated with epileptic discharge over the left temporal region on scalp EEG (top left). The hemodynamic response involved a widespread network, with the maximum in the epileptogenic zone (in this case the left hippocampus). The patient underwent a stereo-encephalography study, which confirmed the left hippocampus as the main generator of the seizures (bottom). Red arrows indicate the EEG onset of the seizure.
In a study combining EEG-fMRI and EEG source imaging to better understand the neuronal dynamics of the BOLD response, the maximum BOLD response (either activation or deactivation) was shown to correspond to IED onset, while secondary BOLD clusters were related to propagation91. In another study of patients in whom both EEG-fMRI and intracranial EEG recordings were available, synchronized intracerebral IED activity was found between regions showing a significant BOLD response, demonstrating the existence of an actual neuronally-based interictal epileptic network, and suggesting a role for EEG-fMRI as a non-invasive tool for mapping this network92.
4. Current challenges and future opportunities
Networks can be mapped non-invasively at multiple levels, spanning from local and inter-regional connectivity to whole-brain topological attributes, thus providing a window into the complex patterns of disease effects.
In preclinical models, imaging technologies are rapidly evolving. It is now possible to image functionally connected areas of the brain, their dynamic changes and perturbations. Refinements in application of these techniques permit repetitive, non-invasive measurements that can track changes in connectivity during the disease course93. In the near future real-time whole brain imaging technologies at single cell resolution in awake rodents may be feasible. Such technology will impact greatly on our ability to understand cellular and circuit mechanisms that result in the development of an epileptic network. For technical reasons calcium-based imaging has mainly focused on recording activity in superficial layers of the cortex. A clear advance will be to adapt these techniques to target deeper brain structures such as the hippocampus and thalamus. However, the biggest preclinical challenge will be to image spontaneous seizures in disease relevant models. Spontaneous seizures can be relatively infrequent, just 1–4 a week in some models. A main advance for the future will be to develop technology that allows continuous imaging of brain activity in freely moving rodents prone to spontaneous seizures. Although miniscope calcium imaging33 goes some way towards addressing this, development of less invasive technologies will be beneficial. The future may see a switch from a preference of calcium-sensitive probes to voltage sensitive indicators. Constitutive high expression of calcium indicators can perturb neuronal calcium homeostasis due to alterations in basal calcium buffering within cells17. Voltage sensitive indicators would circumvent this concern. Improvements in signal-to-noise ratios coupled with faster response times for genetically-encoded voltage sensitive indicators may find these probes becoming the preferred choice.
In human epilepsy, imaging studies have unveiled complex patterns of reorganization of structural and functional networks in various syndromes. The majority have remained observational, largely neglecting the modulatory role of the primary epileptogenic structural lesion and mechanisms leading to network reshaping. Importantly, emerging studies suggest that derangements of lesional morphology and architecture may account for aberrant intrinsic functional connectivity94; 95. Cross-domain interactions have also been only rarely assessed. Methodological advances such as network control theory allow addressing structure-function links mechanistically, specifically predicting how the brain moves between functional states drawn from white matter network organization. This framework thus lends a novel perspective to examine structurally-governed macroscale dysfunction observed in epilepsy94. Combination of connectome models together with imaging of structure and function is likely to further our understanding of the associated cognitive and comorbid psychiatric dysfunction prevalent in many epilepsy syndromes.
A pivotal property of the human connectome is to support efficient communication and integration of information. Methods from network science are thus expanding in new directions, going beyond description of topology towards addressing dynamics, a concept building on the notion that physiological activity of neural systems is constrained by the patterns of connections96. To date, brain communication models have been mainly derived from diffusion-weighted MRI data estimating white matter tracts; most metrics have been designed to quantify information flow along shortest paths, a mode of communication relying exclusively on a small fraction of high-strength connections. Communicability of complex networks may, however, be a broader measure, capturing information flow along all possible paths between any two nodes. Network communication dynamics might be of fundamental importance for understanding plasticity and resilience to disease-related damage, and communicability metrics may indeed be more sensitive to organizational changes than standard connectivity measures. Another line of research in generative models currently operating on intracranial EEG and connectomes derived from diffusion-weighted MRI to study patterns of seizure spread97. However, due to the limited and partial sampling of intracranial EEG, it is imperative to validate these models with non-invasive, whole-brain electrophysiological techniques and integrating them with advanced structural and functional MRI. A coherent multidisciplinary approach will help determining whether a connectome-based mapping of the epileptogenic network is clinically relevant, particularly in relation to surgery. It may also help defining the role of novel anti-seizure approaches still in their infancy, such as gene therapy98, optogenetics99 or chemogenetics100.
Supplementary Material
Key Bullet points:
Epilepsy can be viewed as a system disorder with abnormal network interactions and connectivity at short and long range.
Advances in rodent in vivo imaging allow unprecedented insight into ictogenesis at multiple scales, from neuronal microcircuits to brain-wide networks.
In vivo human imaging combined with network neuroscience has shown a modulatory role of structural anomalies of the primary epileptogenic lesion on local- and large-scale networks.
Acknowledgements:
R.C. Wykes was supported by an Epilepsy Research UK Fellowship (F1401). H.M Khoo was supported by Mark Rayport and Shirley Ferguson Rayport Clinical Fellowship in Epilepsy Surgery and the Preston Robb Fellowship of the Montreal Neurological Institute (Canada), research fellowship of the Uehara Memorial Foundation (Japan), travel grants from Osaka Medical Research Foundation for Intractable Diseases (Japan) and Japan Epilepsy Research Foundation (Japan). L Caciagli is funded by a PhD scholarship from Brain Research UK. P Golshani received grant support from NIH R01 NS099137. J Kapur received grant support from NIH R01 NS040337. A Bernasconi and N. Bernasconi were funded by the Canadian Institute of Health Research (CIHR, MOP-57840 and 123520).
Footnotes
Disclosure
None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References.
- 1.Johnson MR, Shkura K, Langley SR, et al. Systems genetics identifies a convergent gene network for cognition and neurodevelopmental disease. Nat Neurosci 2016;19:223–232. [DOI] [PubMed] [Google Scholar]
- 2.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43:219–227. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt BC, Hong S, Bernasconi A, et al. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 2013;7:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernasconi A Connectome-based models of the epileptogenic network: a step towards epileptomics? Brain 2017;140:2525–2527. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet EA, Dedeurwaerdere S, Cole AJ, et al. WONOEP appraisal: Imaging biomarkers in epilepsy. Epilepsia 2017;58:315–330. [DOI] [PubMed] [Google Scholar]
- 6.Viventi J, Kim DH, Vigeland L, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 2011;14:1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsaki G Large-scale recording of neuronal ensembles. Nat Neurosci 2004;7:446–451. [DOI] [PubMed] [Google Scholar]
- 8.Merricks EM, Smith EH, McKhann GM, et al. Single unit action potentials in humans and the effect of seizure activity. Brain 2015;138:2891–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med 2001;7:1063–1067. [DOI] [PubMed] [Google Scholar]
- 10.Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci 2016;19:1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madisen L, Garner AR, Shimaoka D, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 2015;85:942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017;20:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dana H, Mohar B, Sun Y, et al. Sensitive red protein calcium indicators for imaging neural activity. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima R, Jung A, Yoon BJ, et al. Optogenetic Monitoring of Synaptic Activity with Genetically Encoded Voltage Indicators. Front Synaptic Neurosci 2016;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron 2012;73:862–885. [DOI] [PubMed] [Google Scholar]
- 16.Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013;499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinmetz NA, Buetfering C, Lecoq J, et al. Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines. eNeuro 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenthner CJ, Miyamichi K, Yang HH, et al. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 2013;78:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nat Methods 2013;10:508–513. [DOI] [PubMed] [Google Scholar]
- 20.Yang B, Treweek JB, Kulkarni RP, et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 2014;158:945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aswendt M, Schwarz M, Abdelmoula WM, et al. Whole-Brain Microscopy Meets In Vivo Neuroimaging: Techniques, Benefits, and Limitations. Mol Imaging Biol 2017;19:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Yuste R. In vivo imaging of neural activity. Nat Methods 2017;14:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 2013;4:2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu FH, Mantegazza M, Westenbroek RE, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 2006;9:1142–1149. [DOI] [PubMed] [Google Scholar]
- 25.Muldoon SF, Villette V, Tressard T, et al. GABAergic inhibition shapes interictal dynamics in awake epileptic mice. Brain 2015;138:2875–2890. [DOI] [PubMed] [Google Scholar]
- 26.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol 2003;2:725–730. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel M, Hamm JP, Peterka DS, et al. Reliable and Elastic Propagation of Cortical Seizures In Vivo . Cell Rep 2017;19:2681–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liou JY, Ma H, Wenzel M, et al. Role of inhibitory control in modulating focal seizure spread. Brain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coulter DA, Steinhauser C. Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med 2015;5:a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baird-Daniel E, Daniel AGS, Wenzel M, et al. Glial Calcium Waves are Triggered by Seizure Activity and Not Essential for Initiating Ictal Onset or Neurovascular Coupling. Cereb Cortex 2017;27:3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel AG, Laffont P, Zhao M, et al. Optical electrocorticogram (OECoG) using wide-field calcium imaging reveals the divergence of neuronal and glial activity during acute rodent seizures. Epilepsy Behav 2015;49:61–65. [DOI] [PubMed] [Google Scholar]
- 32.Rossi LF, Wykes RC, Kullmann DM, et al. Focal cortical seizures start as standing waves and propagate respecting homotopic connectivity. Nat Commun 2017;8:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh KK, Burns LD, Cocker ED, et al. Miniaturized integration of a fluorescence microscope. Nat Methods 2011;8:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tervo DG, Hwang BY, Viswanathan S, et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016;92:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuman T, Aharoni D, Cai DJ, et al. Breakdown of spatial coding and neural synchronization in epilepsy. bioRxiv 2018. [Google Scholar]
- 36.Guo JN, Kim R, Chen Y, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol 2016;15:1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoo HM, Hao Y, von Ellenrieder N, et al. The hemodynamic response to interictal epileptic discharges localizes the seizure-onset zone. Epilepsia 2017;58:811–823. [DOI] [PubMed] [Google Scholar]
- 38.Schridde U, Khubchandani M, Motelow JE, et al. Negative BOLD with large increases in neuronal activity. Cereb Cortex 2008;18:1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AJ, Blumenfeld H, Behar KL, et al. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A 2002;99:10765–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englot DJ, Modi B, Mishra AM, et al. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci 2009;29:13006–13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron 2015;85:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furman M, Zhan Q, McCafferty C, et al. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: Effects on cortical physiology. Epilepsia 2015;56:e198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kundishora AJ, Gummadavelli A, Ma C, et al. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation. Cereb Cortex 2017;27:1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeSalvo MN, Schridde U, Mishra AM, et al. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage 2010;50:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra AM, Ellens DJ, Schridde U, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci 2011;31:15053–15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chahboune H, Mishra AM, DeSalvo MN, et al. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage 2009;47:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dezsi G, Ozturk E, Stanic D, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 2013;54:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra AM, Bai X, Motelow JE, et al. Increased resting functional connectivity in spike-wave epilepsy in WAG/Rij rats. Epilepsia 2013;54:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Luijtelaar G, Mishra AM, Edelbroek P, et al. Anti-epileptogenesis: Electrophysiology, diffusion tensor imaging and behavior in a genetic absence model. Neurobiol Dis 2013;60:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernasconi A, Bernasconi N, Bernhardt BC, et al. Advances in MRI for ‘cryptogenic’ epilepsies. Nat Rev Neurol 2011;7:99–108. [DOI] [PubMed] [Google Scholar]
- 51.Bernasconi N, Bernasconi A. Epilepsy: Imaging the epileptic brain--time for new standards. Nat Rev Neurol 2014;10:133–134. [DOI] [PubMed] [Google Scholar]
- 52.Hong SJ, Bernhardt BC, Gill RS, et al. The spectrum of structural and functional network alterations in malformations of cortical development. Brain 2017;140:2133–2143. [DOI] [PubMed] [Google Scholar]
- 53.Bernhardt BC, Bernasconi N, Concha L, et al. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776–1784. [DOI] [PubMed] [Google Scholar]
- 54.Bonilha L, Helpern JA, Sainju R, et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 2013;81:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caciagli L, Bernhardt BC, Hong SJ, et al. Functional network alterations and their structural substrate in drug-resistant epilepsy. Front Neurosci 2014;8:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage 2008;42:515–524. [DOI] [PubMed] [Google Scholar]
- 57.Bernhardt BC, Bernasconi N, Kim H, et al. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012;78:129–136. [DOI] [PubMed] [Google Scholar]
- 58.Concha L, Kim H, Bernasconi A, et al. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 2012;79:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller SS, Richardson MP, Schoene-Bake JC, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol 2015;77:760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaessen MJ, Braakman HM, Heerink JS, et al. Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cereb Cortex 2013;23:1997–2006. [DOI] [PubMed] [Google Scholar]
- 61.van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci 2011;31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Heuvel MP, Kahn RS, Goni J, et al. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci U S A 2012;109:11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Senden M, Deco G, de Reus MA, et al. Rich club organization supports a diverse set of functional network configurations. Neuroimage 2014;96:174–182. [DOI] [PubMed] [Google Scholar]
- 64.Bernhardt BC, Bernasconi N, Hong SJ, et al. Subregional Mesiotemporal Network Topology Is Altered in Temporal Lobe Epilepsy. Cereb Cortex 2016;26:3237–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Besson P, Dinkelacker V, Valabregue R, et al. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage 2014;100:135–144. [DOI] [PubMed] [Google Scholar]
- 66.Chiang S, Stern JM, Engel J Jr., et al. Differences in graph theory functional connectivity in left and right temporal lobe epilepsy. Epilepsy Res 2014;108:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaughan DN, Rayner G, Tailby C, et al. MRI-negative temporal lobe epilepsy: A network disorder of neocortical connectivity. Neurology 2016;87:1934–1942. [DOI] [PubMed] [Google Scholar]
- 68.Bernhardt BC, Bernasconi A, Liu M, et al. The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Ann Neurol 2016;80:142–153. [DOI] [PubMed] [Google Scholar]
- 69.van Diessen E, Zweiphenning WJ, Jansen FE, et al. Brain Network Organization in Focal Epilepsy: A Systematic Review and Meta-Analysis . PLoS One 2014;9:e114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiang S, Stern JM, Engel J Jr., et al. Structural-functional coupling changes in temporal lobe epilepsy. Brain Res 2015;1616:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bettus G, Guedj E, Joyeux F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 2009;30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maccotta L, He BJ, Snyder AZ, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin 2013;2:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haneef Z, Lenartowicz A, Yeh HJ, et al. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014;55:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He X, Doucet GE, Sperling M, et al. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia 2015;56:1571–1579. [DOI] [PubMed] [Google Scholar]
- 75.Stretton J, Winston GP, Sidhu M, et al. Disrupted segregation of working memory networks in temporal lobe epilepsy. Neuroimage Clin 2013;2:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voets NL, Beckmann CF, Cole DM, et al. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain 2012;135:2350–2357. [DOI] [PubMed] [Google Scholar]
- 77.Voets NL, Zamboni G, Stokes MG, et al. Aberrant functional connectivity in dissociable hippocampal networks is associated with deficits in memory. J Neurosci 2014;34:4920–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu M, Bernhardt BC, Hong SJ, et al. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain 2016;139:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao F, Caciagli L, Wandschneider B, et al. Effects of carbamazepine and lamotrigine on functional magnetic resonance imaging cognitive networks. Epilepsia 2018;59:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caciagli L, Xiao F, Wandschneider B, et al. Imaging Biomarkers of Anti-Epileptic Drug Action: Insights from Magnetic Resonance Imaging. Curr Pharm Des 2017;23:5727–5739. [DOI] [PubMed] [Google Scholar]
- 81.An D, Fahoum F, Hall J, et al. Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy. Epilepsia 2013;54:2184–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pittau F, Grova C, Moeller F, et al. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 2012;53:1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tousseyn S, Dupont P, Goffin K, et al. Sensitivity and Specificity of Interictal EEG-fMRI for Detecting the Ictal Onset Zone at Different Statistical Thresholds. Front Neurol 2014;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fahoum F, Lopes R, Pittau F, et al. Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia 2012;53:1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Federico P, Archer JS, Abbott DF, et al. Cortical/subcortical BOLD changes associated with epileptic discharges: an EEG-fMRI study at 3 T. Neurology 2005;64:1125–1130. [DOI] [PubMed] [Google Scholar]
- 86.Flanagan D, Badawy RA, Jackson GD. EEG-fMRI in focal epilepsy: local activation and regional networks. Clin Neurophysiol 2014;125:21–31. [DOI] [PubMed] [Google Scholar]
- 87.Luo C, An D, Yao D, et al. Patient-specific connectivity pattern of epileptic network in frontal lobe epilepsy. Neuroimage Clin 2014;4:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One 2011;6:e20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhary UJ, Carmichael DW, Rodionov R, et al. Mapping preictal and ictal haemodynamic networks using video-electroencephalography and functional imaging. Brain 2012;135:3645–3663. [DOI] [PubMed] [Google Scholar]
- 90.Donaire A, Bargallo N, Falcon C, et al. Identifying the structures involved in seizure generation using sequential analysis of ictal-fMRI data. Neuroimage 2009;47:173–183. [DOI] [PubMed] [Google Scholar]
- 91.Vulliemoz S, Thornton R, Rodionov R, et al. The spatio-temporal mapping of epileptic networks: combination of EEG-fMRI and EEG source imaging. Neuroimage 2009;46:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khoo HM, von Ellenrieder N, Zazubovits N, et al. Epileptic networks in action: Synchrony between distant hemodynamic responses. Ann Neurol 2017;82:57–66. [DOI] [PubMed] [Google Scholar]
- 93.Bertoglio D, Jonckers E, Ali I, et al. In vivo measurement of brain network connectivity reflects progression and intrinsic disease severity in a model of temporal lobe epilepsy. Neurobiol Dis 2019;127:45–52. [DOI] [PubMed] [Google Scholar]
- 94.Bernhardt BC, Fadaie F, Vos de Wael R, et al. Preferential susceptibility of limbic cortices to microstructural damage in temporal lobe epilepsy: A quantitative T1 mapping study. Neuroimage 2018;182:294–303. [DOI] [PubMed] [Google Scholar]
- 95.Hong SJ, Lee HM, Gill R, et al. A connectome-based mechanistic model of focal cortical dysplasia. Brain 2019;142:688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci 2017;19:17–33. [DOI] [PubMed] [Google Scholar]
- 97.Proix T, Bartolomei F, Guye M, et al. Individual brain structure and modelling predict seizure propagation. Brain 2017;140:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kullmann DM, Schorge S, Walker MC, et al. Gene therapy in epilepsy-is it time for clinical trials? Nat Rev Neurol 2014;10:300–304. [DOI] [PubMed] [Google Scholar]
- 99.Wykes RC, Kullmann DM, Pavlov I, et al. Optogenetic approaches to treat epilepsy. J Neurosci Methods 2016;260:215–220. [DOI] [PubMed] [Google Scholar]
- 100.Katzel D, Nicholson E, Schorge S, et al. Chemical-genetic attenuation of focal neocortical seizures. Nat Commun 2014;5:3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.