Figure 2.
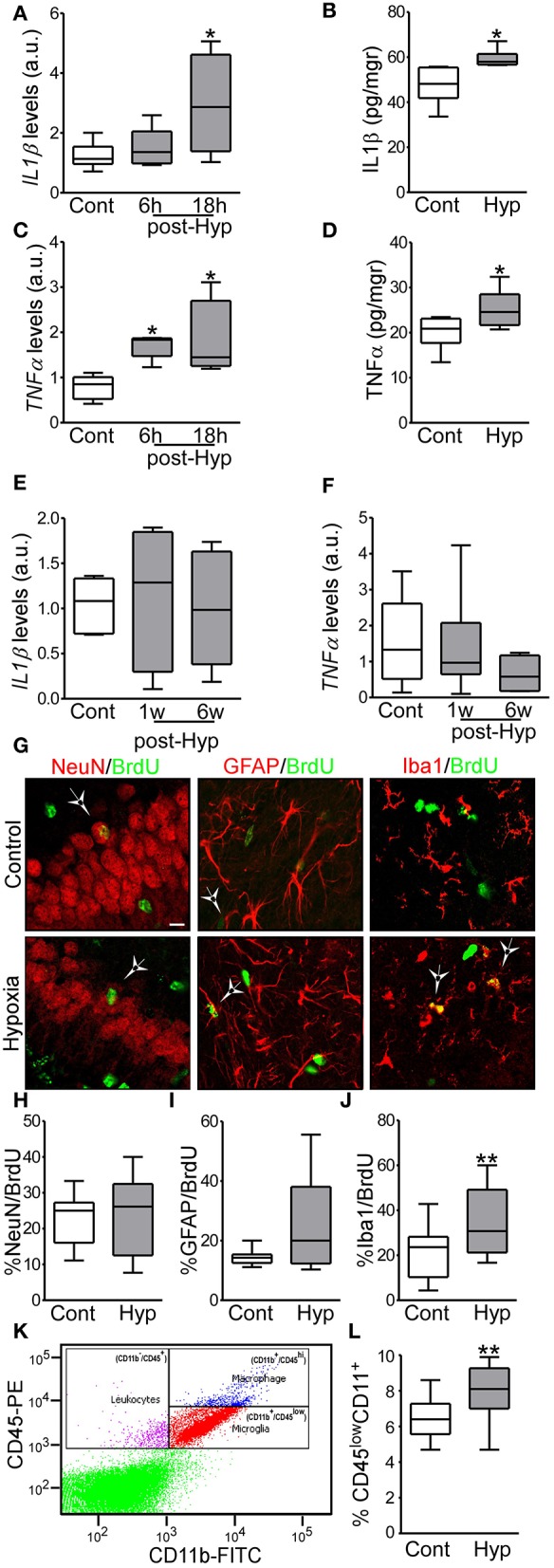
Hypoxia-induced seizures induce acute inflammation and microglia proliferation in a neonatal mouse model. (A,B) IL1β mRNA and protein levels are increased acutely in hypoxia-exposed pups compared to control [n = 6 per group. *p < 0.05. A, F: 4.854, t: 3.028 (vs. control), t: 0.568 (vs. 6 h). B, F: 4.477, t: 2.485)] (C,D) TNFα mRNA and protein levels are increased acutely in the hippocampus in hypoxia exposed mice compared to control [n = 6 per group. *p < 0.05. C, F: 6.736, t: 2.975 (vs. control), t: 2.138 (vs. 6 h). D, F: 1.322, t: 2.260)]. (E,F) Levels of IL1β and TNFα were normalized at 1 week and 6 weeks after hypoxia (n = 6 per group). (G) Representative confocal images of double-staining cells with BrdU and NeuN (neuronal marker, NeuN/BrdU) or GFAP (astrocytic marker, GFAP/BrdU) or Iba1 (microglia marker, Iba1/BrdU) in the hippocampus 72 h post hypoxia. Scale bar: 10 μm. (H–J) Quantification of the percentage of BrdU positive cells are also positive for NeuN (H), GFAP (I) or Iba 1 (J) (n = 16 per group. **p < 0.01. J, F: 1.471, t: 3.133). (K) Representative scatter plot shows the distribution of CD45 and CD11 from control brain. (L) Quantitative analysis of the percentage of CD11+/CD45low as a representation of the microglia population in control and hypoxia exposed pups (n = 6 per group. **p < 0.01. F: 2.397, t: 3.831).
