Figure 5.
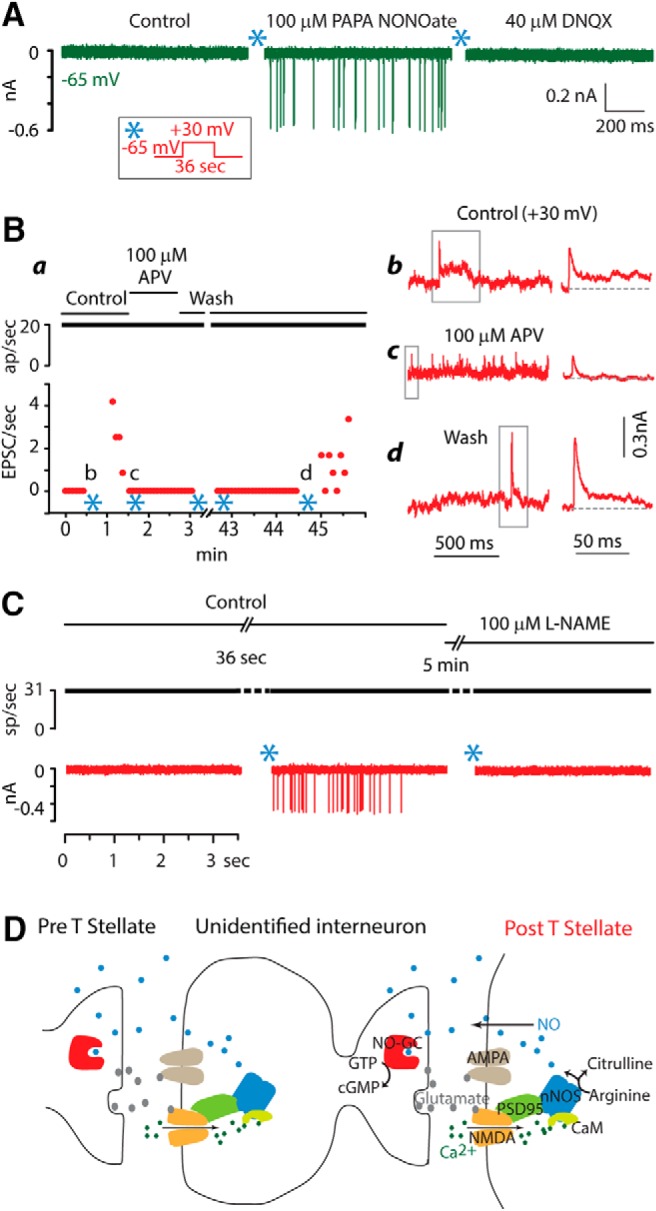
Pharmacological studies support a role of NO signaling in potentiation of interconnections between T-stellate cells. A, Application of an NO donor, PAPA NONOate, mimicked potentiation in T-stellate cells. It evoked EPSCs of approximately uniform amplitude that were mediated through AMPA receptors. B, Potentiation requires NMDA receptors. Ba, Plot summarizes one dual recording. The presynaptic cell fired constantly throughout the experiment. Initially no EPSCs were observed. After pairing presynaptic firing with postsynaptic depolarization (*), EPSCs were initially rapid and over ∼1 min, the frequency dropped. The application of APV blocked potentiation and also reversibly eliminated slow components of EPSCs visible while the cell was depolarized to +30 mV. Bb–Bd, Traces on the left, shown after smoothing, were recorded while the cell was depolarized to +30 mV at the times indicated in a. During depolarization to +30 mV, EPSCs were reversed. Panels on the right show EPSCs marked by gray boxes on the left at expanded time scales. EPSCs had early rapid components that were sometimes followed by a slower component (Bb, Bd). In the presence of of APV, the rapid phases remained but no long EPSCs were observed (Bc). C, Potentiation depended on nNOS. Pairing of presynaptic firing at 33/s with a postsynaptic depolarization to +30 mV for 36 s (*) evoked EPSCs. The subsequent addition to the bath of L-NAME, NG-nitro-l-argenine methyl ester, a blocker of nNOS, prevented potentiation. This experiment was repeated in 3 cells. D, These results are consistent with potentiation being mediated through a signaling pathway such as that proposed by Boehning and Snyder (Boehning and Snyder, 2003). Release of glutamate from presynaptic terminals activates postsynaptic AMPA and NMDA receptors. Ca2+ entering through NMDA receptors binds calmodulin (CaM) which in turn activates neuronal nitric oxide synthase (nNOS). nNOS converts arginine to citrulline and NO. Being a small gas, NO diffuses not just within the cell but also through the membrane to adjacent cells. The NO receptor guanylate cyclase (NO-GC) in presynaptic terminals detects NO and raises levels of cGMP. Whether NO signaling acts at only one of the synapses or at all synapses in a polysynaptic pathway is not known. Possibly NO generated at multiple synapses sums to influence other nearby synapses. NO can also act within the cell in which it is synthesized on targets with lower affinities to affect the trafficking of AMPA receptors (Bradley and Steinert, 2016).
