Abstract
While diarrhea mortality in children has declined over the last two decades, there has been a slower decline in diarrheal episodes. Repeated diarrheal episodes are associated with childhood stunting, which leads to increased mortality risk from infectious diseases. Vaccine candidates are under development for enterotoxigenic Escherichia coli [ETEC] and Shigella, important enteric pathogens in children in low income countries. These future vaccines could significantly reduce diarrheal burden, prevent ETEC- and Shigella-induced stunting, and stunting-associated mortality.
We developed a cost-effectiveness model for two putative standalone ETEC and Shigella vaccine candidates to evaluate vaccine impact on mortality, morbidity, stunting, and stunting-associated deaths from other infectious diseases. We modeled impact over the first ten years after vaccine introduction in children under five years old living in 79 low and low-middle income countries.
ETEC and Shigella diarrhea would cause an estimated 239,300 [95% UL: 179,700–309,800] and 340,300 [256,500–440,800] child deaths, respectively, from years 2025 to 2034. Most of these deaths would occur in AFRO countries. ETEC and Shigella moderate-to-severe diarrheal episodes would result in over 13.7 [8.4–19.0] and 21.4 [13.1–29.8] million stunted children, respectively. Introducing ETEC or Shigella vaccine each with 60% efficacy could prevent 92,000 [61,000–129,000] ETEC and 126,600 [84,000–179,000] Shigella direct deaths and 21,400 [11,300–34,800] ETEC- and 34,200 [18,000–56,000] Shigella-induced stunting deaths. ETEC ICERs ranged from $2172/DALY [1457–4369] in AFRO to $19,172/DALY [12,665–39,503] in EURO. Shigella ICERs ranged from $952/DALY [632–2001] in EMRO to $640,316/DALY [434,311–1,297,192] in EURO.
Limitations of this analysis include uncertainty of vaccine efficacy, duration of protection, and vaccine price. Inclusion of other infectious disease mortality due to stunting provides a more accurate assessment of total ETEC and Shigella disease burden and increased the projected impact and cost-effectiveness of vaccination. Introducing vaccines only in high burden countries and regions could substantially reduce cost without substantially reducing impact.
Keywords: ETEC, Shigella, Diarrhea, Stunting, Cost-effectiveness, Vaccines
1. Introduction
Globally, diarrhea remains the second leading cause of mortality, accounting for approximately 500,000 deaths annually in children under five years old [1]. However, diarrheal mortality in children has declined by 34.3%, with similar declines in Shigellosis (33.8%) and enterotoxigenic E. coli (ETEC) infection (38.1%) from 2005 to 2015 [2]. While diarrheal mortality has declined, morbidity and mortality continue to plague many low- and lower middle-income countries (LMICs).
In addition to rotavirus, other pathogens have a substantial role in diarrheal burden. The Global Enteric Multicenter Study (GEMS) found that of 22 diarrheal pathogens, four—rotavirus, Shigella, ETEC, and Cryptosporidium—were associated with moderate-to-severe diarrhea (MSD), accounting for 70% of cases in 0–4 year olds [3]. This study also documented an increased mortality risk for MSD ETEC cases and increased stunting risk in cases associated with both ETEC and Shigella [4]. ETEC and Shigella have been among the top four causes of diarrhea associated with years lost to disability (YLDs) worldwide [5]. A recent study evaluating global ETEC and Shigella burden found that these pathogens have a sizeable global burden, especially in the World Health Organization (WHO) designated African and the Eastern Mediterranean regions [6].
As diarrheal mortality has declined, there is increased focus on diarrhea morbidity in pediatric populations. Repeated, non-fatal episodes of diarrhea from infection by certain pathogens are thought responsible for reduced linear growth and childhood stunting [7], [8], [9], increasing mortality risk from other infectious diseases [10]. Results from GEMS showed that MSD episodes shifted the height-for-age z-scores downward [3], [11], increasing a child’s risk of stunting. In countries where stunting is highly prevalent, ETEC- and Shigella-induced stunting impacts even more children [12].
The burden from these pathogens necessitates new prevention strategies. A potential and highly beneficial prevention strategy would be the use of vaccines to prevent ETEC and Shigella infection, which are currently under development with Phase 1 and Phase 2 data available shortly in non-infant populations. In addition to reducing burden worldwide, the successful rollout of rotavirus vaccines has shown that enteric vaccines are deployable to endemic countries. Studies describing rotavirus burden, vaccine impact, and cost-effectiveness have contributed to global and country decisions to accelerate rotavirus vaccine introduction [13], [14]. Therefore, investigating how ETEC and Shigella vaccination could impact high burden countries or populations is not only important in guiding vaccination programs, but may in turn spur action by policymakers.
In this study, we conducted a vaccine impact and cost-effectiveness analysis for ETEC and Shigella vaccines to identify high-need areas and to capture the full potential value of these vaccines. We evaluated the impact of vaccination on the mortality, morbidity, number of stunted children, and stunting-associated deaths from other infectious diseases, to understand the expanded impact of these vaccines.
2. Materials and methods
2.1. Population and time frame
We included seventy-nine countries from a previous analysis of ETEC and Shigella burden with a full description of country inclusion and exclusion criteria [6] (Table 1). We aggregated national estimates by WHO regions to identify trends (‘AFRO’: African region, ‘AMRO’: Region of the Americas, ‘EMRO’: Eastern Mediterranean Region, ‘SEARO’: Southeast Asian Region, ‘WPRO’: Western Pacific Region). We assumed all countries would introduce the vaccines in 2025. We examined 10 annual birth cohorts of children over the first five years of life. Population estimates of under five children were based on UN Population Division country estimates and projections from 2025 to 2034 [15].
Table 1.
The 79 Countries included in study by WHO region. AFRO: African region, AMRO: Region of the Americas, EMRO: Eastern Mediterranean Region, SEARO: Southeast Asian Region, WPRO: Western Pacific Region.
| AFRO | AMRO | EMRO | EURO | SEARO | WPRO | Excluded | |
|---|---|---|---|---|---|---|---|
| Angola | Liberia | Bolivia | Afghanistan | Armenia | Bangladesh | Cambodia | Cabo Verde |
| Benin | Madagascar | El Salvador | Djibouti | Georgia | Bhutan | Kiribati | Kosovo |
| Burkina Faso | Malawi | Guatemala | Egypt | Kyrgyz Republic | DPR Korea | Lao PDR | Micronesia |
| Burundi | Mali | Haiti | Jordan | Moldova | India | Mongolia | Moldova |
| Cameroon | Mauritania | Honduras | Morocco | Tajikistan | Indonesia | Papua New Guinea | Vanuatu |
| Central African Republic | Mozambique | Nicaragua | Pakistan | Ukraine | Myanmar | Philippines | |
| Chad | Niger | Somalia | Uzbekistan | Nepal | Solomon Islands | ||
| Comoros | Nigeria | Sudan | Sri Lanka | Viet Nam | |||
| Congo | Rwanda | Syria | Timor-Leste | ||||
| Congo DR | São Tomé & Principe | Tunisia | |||||
| Côte d’Ivoire | Senegal | Yemen | |||||
| Eritrea | Sierra Leone | ||||||
| Ethiopia | South Sudan | ||||||
| The Gambia | Swaziland | ||||||
| Ghana | Tanzania | ||||||
| Guinea | Togo | ||||||
| Guinea-Bissau | Uganda | ||||||
| Kenya | Zambia | ||||||
| Lesotho | Zimbabwe | ||||||
2.2. Burden of diarrhea mortality and morbidity
2.2.1. Etiological fraction
ETEC and Shigella burden is dependent upon the fraction of episodes and deaths attributable to each [3], [16]. As previously described [6], we used culture-based etiological estimates [3] and adjusted them for under-detection using estimates derived from molecular methods based on Liu et al. [17], [18]. We applied an adjustment of 1.5 times for ETEC and 2.0 times for Shigella to the culture results [20].
2.2.2. Diarrhea mortality and morbidity estimates
Similar to our burden study, we used the mid-point of 2015 diarrheal mortality estimates from two sources: the Global Burden of Disease study (GBD) at the Institute for Health Metrics and Evaluation (IHME) [19] and the WHO Maternal Child Epidemiology Estimation (MCEE) project [20]. We then used the etiological fraction for ETEC and Shigella to calculate pathogen-attributable deaths and adjusted diarrheal mortality to account for countries that introduced rotavirus vaccine before 2014 [6]. We projected mortality and morbidity estimates from 2015 to 2034. Because diarrheal mortality rates have declined over time, we estimated annual rates of decline for non-rotavirus under-five diarrheal mortality in each country using data from Child Health Epidemiology Reference Group from 2000 to 2013 [21]. We calculated diarrhea morbidity estimates using WHO region-specific estimates of diarrhea episodes and the etiological fractions for ETEC and Shigella [22]. We assumed morbidity declined at a rate of 0.45% per year. This decline percentage was calculated from YLDs from diarrheal disease from 1990 to 2010 from the GBD [23].
2.2.3. Effects of ETEC- and Shigella-induced stunting
We applied the methods from Anderson et al. [6] to determine the effects of ETEC and Shigell-induced stunting. First, we calculated the shift in child height-for-age z-scores from ETEC and Shigella episodes using GEMS results (Table 1). In the absence of reliable community-level estimates for diarrhea treatment in countries included in this study, we assumed 22% of diarrheal episodes where care was sought at a health facility were considered MSD. The proportion of child diarrheal episodes where caretakers sought care were taken from the most recent Demographic and Health Surveys (DHS), available for 70 countries [24]. In countries without a country-level estimate, we substituted the corresponding WHO regional average. Based on the mean of estimates for the countries included in this analysis, we calculated that 47% of caretakers would visit a health facility after the child experienced an episode of diarrhea (Table 1). Thus, we assume that 10% (22% × 47%) of child diarrheal episodes were moderate-to-severe in each region. We used the same the approach as in Anderson et al. [6] to estimate the number of child deaths from infections for which stunting is a risk factor (pneumonia, malaria, measles, and diarrhea [25]). We did not project rate of change of pneumonia, malaria and measles burden over time.
2.3. Outcomes measures
Outcome measures included: diarrheal episodes; direct deaths; children stunted; stunted children dying from other infectious diseases; and Disability-adjusted Life Years (DALYs). Stunting is a risk factor for other infectious disease deaths and not directly included in DALY calculations. All mortality outcomes, including other infectious disease deaths due to induced stunting, were translated to DALYs using standard techniques [26], [27]. We calculated DALYs using non-uniform age-weighting and a 3% annual discount rate. We calculated all outcomes annually and cumulatively from time of introduction.
2.4. Vaccines
We evaluated the impact of potential ETEC and a Shigella standalone vaccine candidates when introduced nationally in 79 countries. We assume each vaccine is 60% efficacious in preventing deaths and MSD episode. Our other assumptions were that protection was conferred after the third dose and that there was no protection for partially vaccinated children. We assumed that vaccine effectiveness does not wane, but we also assumed that there was no effectiveness after five years of age or herd protection. For coverage, we used country specific 2015 DPT3 coverage estimates [28] and only included estimates for children estimated to receive a full course (all three doses).
As vaccine price is uncertain, we used the Gavi Rotarix price of approximately $2.00 per dose [29] as our basis. For our study, we assumed study vaccines would cost $3.30 per dose, and we varied this price in the sensitivity analysis to assess price impact on cost-effectiveness.
2.5. Costs
As there are no published medical costs for ETEC and Shigella diarrhea for the 79 study countries, we used country specific estimates of direct medical costs of illness associated with inpatient and outpatient care for MSD episodes. We assumed 1 of 8 outpatients with MSD were referred for inpatient care [13], [30].
Direct medical costs were based on WHO-CHOICE Service Delivery Unit Cost estimates and commodity costs. For outpatient medical costs, we used country specific cost per outpatient visit at a primary hospital. We used country specific daily costs at hospitals and a four-day stay to calculate inpatient cost. We assumed that outpatients receive six oral rehydration solution packets per day for two days, and inpatients receive six packets per day and two IVs during a four day hospital stay [31]. On average, the illness cost per episode was $10.05 for outpatients and $82.25 for inpatients. These estimates were triangulated against country specific estimates, when available in the literature [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]. In most cases, modelled estimates were aligned with empirical estimates.
Using data available from Portnoy et al. [48], we generated vaccine administration costs. All costs were in 2016 US dollars and discounted (3%). Our cost-effectiveness estimates were from the health system perspective.
2.6. Cost-effectiveness
We calculated vaccination cost () cumulated over the first 10 years () after introduction starting in 2025 for each country () based on vaccine administration cost, vaccine price, and quantity (birth cohort times coverage rate with 10% vaccine wastage). We calculated averted costs () based on population, vaccine coverage, efficacy, and access to care and medical costs in each country (). We calculated net costs () for each region () as:
We calculated vaccine benefits () for each region () based on the sum of population, coverage (), efficacy (), and DALY burden () in each country () cumulated over the first 10 years () after introduction. We calculated the number of children fully vaccinated each year by multiplying the annual birth cohort by the assumed vaccine coverage. We projected benefits for the first five years of life for children vaccinated in each annual birth cohort.
Our primary cost-effectiveness measure was the regional Incremental Cost-Effectiveness Ratio (), which is the aggregated country-level incremental costs associated with introducing each vaccine divided by aggregated country-level health benefit.
Our comparator scenario was no vaccination. We calculated ICERS with and without other infectious disease burden due to stunting for each country and region annually and cumulatively.
We presented results using two thresholds for cost-effectiveness as compared to GDP [49]. We presented results for countries where ICERS are less than 3 times their GDP, which are considered cost effective and results for countries where ICERS are less than GDP, which has historically been used to determine if an intervention is ‘highly’ cost-effective.
2.7. Sensitivity analysis
In order to assess the impact of uncertainty and changes in key input variables, we conducted two types of sensitivity analysis using SimVoi [50]. First, we used one-way sensitivity analysis to demonstrate the impact of individual input parameters on vaccination cost-effectiveness. These results are shown in tornado diagrams, with each horizontal band showing the effect of varying each parameter between high and low values. Second, we conducted a probabilistic sensitivity analysis (PSA) to show the overall impact of input parameter uncertainty (Table 2) on our estimates of key outcomes. Monte Carlo analysis using 10,000 iterations was conducted and we included estimated upper and lower 95% uncertainty limits (2.5% and 97.5%) for key outputs in brackets after our estimates. We reported results of the sensitivity analyses as a range of the difference between high and low ICER estimates.
Table 2.
Model parameters for base case scenario and ranges used in uncertainty and sensitivity analyses.
| Model input | Values | Range | Reference |
|---|---|---|---|
| Burden | |||
| Population estimates | Varies by country | – | [17] |
| Diarrheal mortality for children under 5 years of age | Varies by country | ±10%; Triangular | [1], [22], [25] |
| Change in non-rotavirus under-5 diarrheal mortality rate | Varies by country; mean = 12.8% decline | ±25%; Triangular | [23] |
| Diarrheal episodes in children under 5 years of age | Varies by region; 2.2–3.3 episodes/child annually | – | [6], [56] |
| Etiological fraction attributed to ETEC by WHO region | Varies by WHO region; 0.075–0.123 | ±25%; Triangular | [20], [24] |
| Etiological fraction attributed to Shigella by WHO region | Varies by WHO region; 0.002–0.238 | ±25%; Triangular | [20], [24] |
| Stunting induced by ETEC episodes | 0.068 shift in HAZ | ±50%; Triangular | [3], [6] |
| Stunting induced by Shigella episodes | 0.082 shift in HAZ | ±50%; Triangular | [3], [6] |
| Fraction of diarrhoeal episodes that are moderate to severe | 22% of children who sought care at a healthcare facility | 0.12–0.32; Triangular | [3], [6] |
| Percentage of caretakers that sought care at a health facility after a child’s diarrheal episode | 47% | 44–58%, Not varied in uncertainty analysis | [24] |
| Vaccination and Medical Costs | |||
| ETEC vaccine efficacy | 60% | ±20%; Triangular | Assumption |
| Shigella vaccine efficacy | 60% | ±20%; Triangular | Assumption |
| Dose price | $3.30 | $2.50–$7.00; Triangular | Assumption |
| Administration cost | LI = $1.93, LMI = $1.64 | ±40%; Triangular | [48] |
| Cost of ETEC or Shigella illness | Varies by country; outpatient mean = $6.09/episode; inpatient mean = $51.07/episode | ±40%; Triangular | [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47] |
| Inpatient visit rate | 12.5% of outpatient visits | ±50%; Triangular | [30] |
| Outpatient visit rate (cases taken to healthcare facility) | Varies by country; Mean of 47% ETEC or Shigella cases sought treatment annually | 44–58%, Not varied in uncertainty analysis | [24] |
3. Results
3.1. Expected outcomes over time
Over 10-years in 79 LMICs, ETEC and Shigella would cause an estimated 239,300 [95% CI: 180,100; 310,000] and 340,300 [256,700; 440,800] deaths, respectively, in children under five without vaccination (Table 3, Table 4). Most deaths would occur in AFRO for ETEC (68%) and Shigella (54%). In addition, MSD episodes of ETEC and Shigella would result in 13.7 [8.3; 19.1] and 21.4 [13.2; 29.8] million stunted children, respectively. These cases of stunting result in an additional 45,400 [28,700; 59,800] and 72,900 [47,600; 91,900] deaths due to other infectious diseases indirectly attributable to ETEC and Shigella, respectively. The global burden of ETEC and Shigella in LMICs is estimated at 5.3 [4.0; 6.8] and 7.5 [5.6; 9.7] deaths/100,000 children, respectively, with the highest rates in EMRO (Shigella) and AFRO (ETEC) regions. Globally, ETEC would cause 93 [90; 96] million MSD episodes while Shigella would cause 118 [115; 121] million MSD episodes (Table 2, Table 3). AFRO accounts for 49% and 43% of global ETEC and Shigella episodes, respectively, followed by SEARO at 28% (ETEC) and EMRO at 32% (Shigella).
Table 3.
Estimated disease burden associated with ETEC infection and ETEC vaccination impact in children under 5 years of age in 79 countries, by region. Model estimates are projected from 2025 (year of introduction) to 2034. Results from uncertainty analysis are listed below model estimates. Upper and Lower represent 95% uncertainty intervals for each estimate.
| AFRO | AMRO | EMRO | EURO | SEARO | WPRO | GAVI-eligible | Global | |
|---|---|---|---|---|---|---|---|---|
| Number of countries | 38 | 6 | 11 | 7 | 9 | 8 | 48 | 79 |
| ETEC disease burden | ||||||||
| MSD episodes (millions) | 45.81 | 2.25 | 15.00 | 1.15 | 25.64 | 3.00 | 49.59 | 92.84 |
| [44.57; 47.05] | [2.19; 2.31] | [14.31; 15.68] | [1.13; 1.16] | [24.90; 26.38] | [2.95; 3.04] | [48.00; 51.19] | [90.05; 95.64] | |
| ETEC-induced stunting cases (millions) | 6.53 | 0.28 | 2.01 | 0.15 | 4.33 | 0.38 | 7.08 | 13.68 |
| [3.99; 9.08] | [0.17; 0.40] | [1.22; 2.80] | [0.09; 0.21] | [2.64; 6.02] | [0.23; 0.53] | [4.32; 9.85] | [8.35; 19.02] | |
| Total deaths* (1000 s) | 161.92 | 2.37 | 34.98 | 0.48 | 36.85 | 2.65 | 135.62 | 239.25 |
| [121.88; 209.32] | [1.76; 3.09] | [26.51; 44.78] | [0.35; 0.64] | [26.96; 48.88] | [2.00; 3.41] | [102.26; 175.17] | [179.73; 309.81] | |
| Total deaths*/100,000 children** | 8.56 | 3.68 | 5.54 | 0.70 | 2.20 | 1.18 | 6.34 | 5.25 |
| [6.44; 11.07] | [2.74; 4.80] | [4.20; 7.09] | [0.51; 0.93] | [1.61; 2.92] | [0.888; 1.510] | [4.78; 8.19] | [3.94; 6.80] | |
| Other Infectious disease deaths from ETEC-induced stunting as a percentage of total ETEC deaths | 20 | 13 | 19 | 14 | 16 | 14 | 19 | 19 |
| [13; 26] | [8; 17] | [12; 24] | [9; 18] | [10; 21] | [9; 18] | [13; 25] | [12; 25] | |
| Total DALYs* (1000 s) | 5613.1 | 85.3 | 1220.9 | 18.6 | 1302.7 | 97.5 | 4720.2 | 8338.1 |
| [4226.8; 7252.5] | [63.4; 111.3] | [925.3; 1562.5] | [13.6; 24.8] | [953.0; 1728.4] | [73.7; 125.4] | [3559.8; 6093.1] | [6267.0; 10,798.9] | |
| Total DALYS* /100,000 children** | 296.7 | 132.6 | 193.4 | 27.2 | 77.8 | 43.2 | 220.7 | 183.0 |
| [223.4; 383.4] | [98.6; 173.1] | [146.6; 247.5] | [19.8; 36.1] | [56.9; 103.2] | [32.7; 55.6] | [166.4; 284.9] | [137.6; 237.0] | |
| Outpatient treatment costs (millions US$) | 190.8 | 12.5 | 77.1 | 7.7 | 174.8 | 17.4 | 215.2 | 480.1 |
| [131.7; 250.7] | [8.6; 16.4] | [53.2; 101.3] | [5.3; 10.1] | [120.7; 229.6] | [12.0; 22.8] | [148.6; 282.7] | [331.5; 630.9] | |
| Inpatient treatment costs (millions US$) | 160.8 | 15.1 | 76.6 | 10.6 | 207.6 | 20.0 | 161.7 | 490.6 |
| [87.6; 252.8] | [8.2; 23.7] | [41.7; 120.3] | [5.8; 16.7] | [113.0; 326.2] | [10.9; 31.5] | [88.1; 254.2] | [267.2; 771.2] | |
| ETEC vaccination IMPACT | ||||||||
| MSD episodes averted (millions) | 22.61 | 1.21 | 7.10 | 0.57 | 13.77 | 1.72 | 25.45 | 46.97 |
| [16.76; 28.46] | [0.89; 1.52] | [5.26; 8.94] | [0.43; 0.72] | [10.21; 17.34] | [1.27; 2.16] | [18.86; 32.04] | [34.82; 59.14] | |
| ETEC-induced stunting cases averted (millions) | 3.17 | 0.15 | 0.92 | 0.07 | 2.32 | 0.22 | 3.57 | 6.85 |
| [1.81; 4.79] | [0.09; 0.23] | [0.53; 1.39] | [0.04; 0.10] | [1.3; 3.5] | [0.12; 0.33] | [2.03; 5.38] | [3.91; 10.34] | |
| Direct diarrheal deaths averted (1000 s) | 59.31 | 1.01 | 13.44 | 0.24 | 16.73 | 1.24 | 53.72 | 91.97 |
| [39.86; 83.85] | [0.67; 1.45] | [9.06; 18.89] | [0.16; 0.35] | [11.00; 24.17] | [0.83; 1.75] | [36.11; 75.97] | [61.55; 130.39] | |
| Direct diarrheal deaths averted/100,000 FVC*** | 3.80 | 1.75 | 2.47 | 0.44 | 1.10 | 0.60 | 2.85 | 2.33 |
| [2.55; 5.37] | [1.16; 2.50] | [1.66; 3.47] | [0.29; 0.64] | [0.72; 1.59] | [0.40; 0.85] | [1.92; 4.04] | [1.56; 3.31] | |
| Infectious disease deaths due to induced stunting averted (1000 s) | 14.68 | 0.15 | 3.09 | 0.04 | 3.21 | 0.20 | 12.99 | 21.36 |
| [7.89; 23.90] | [0.08; 0.25] | [1.66; 5.00] | [0.02; 0.06] | [1.70; 5.29] | [0.11; 0.32] | [6.98; 21.15] | [11.47; 34.81] | |
| Infectious disease deaths due to induced stunting/100,000 FVC | 0.94 | 0.26 | 0.57 | 0.07 | 0.21 | 0.10 | 0.69 | 0.54 |
| [0.50; 1.53] | [0.14; 0.43] | [0.31; 0.92] | [0.04; 0.11] | [0.11; 0.35] | [0.05; 0.15] | [0.37; 1.12] | [0.29; 0.88] | |
| Total deaths* averted (1000 s) | 73.99 | 1.16 | 16.53 | 0.28 | 19.94 | 1.43 | 66.72 | 113.33 |
| [49.43; 105.71] | [0.77; 1.67] | [11.08; 23.48] | [0.18; 0.41] | [13.07; 29.10] | [0.96; 2.04] | [44.59; 95.28] | [75.49; 162.29] | |
| Total deaths* averted /100,000 FVC*** | 4.74 | 2.01 | 3.04 | 0.51 | 1.31 | 0.69 | 3.54 | 2.87 |
| [3.16; 6.77] | [1.33; 2.89] | [2.04; 4.31] | [0.33; 0.75] | [0.86; 1.92] | [0.463; 0.984] | [2.37; 5.06] | [1.91; 4.11] | |
| Total DALYs* averted (1000 s) | 2532 | 40 | 563 | 9 | 683 | 49 | 2281 | 3876 |
| [1690; 3617] | [26; 57] | [378; 800] | [6; 14] | [448; 997] | [33; 69] | [1524; 3259] | [2580; 5549] | |
| Total DALYS* averted/100,000 FVC*** | 162 | 69 | 104 | 17 | 45 | 24 | 121 | 98 |
| [108; 232] | [46; 99] | [69; 147] | [11; 26] | [29; 66] | [16; 34] | [81; 173] | [65; 141] | |
| Vaccination costs (millions US$) | 5685 | 206 | 1928 | 191 | 5365 | 730 | 6835 | 14,104 |
| [4501; 9204] | [163; 336] | [1523; 3150] | [151; 313] | [4237; 8774] | [576; 1194] | [5412; 11,075] | [11,149; 22,976] | |
| Vaccination costs/100,000 FVC*** | 363,978 | 355,339 | 354,231 | 352,802 | 353,201 | 352,645 | 363,155 | 357,608 |
| [288,159; 589,232] | [280,801; 579,870] | [279,885; 578,803] | [278,605; 577,225] | [278,989; 577,647] | [278,455; 577,074] | [287,524; 588,425] | [282,681; 582,536] | |
| Administration costs/100,000 FVC*** | 105,908 | 98,125 | 97,127 | 95,840 | 96,199 | 95,698 | 105,166 | 100,169 |
| [72,792; 138,495] | [67,442; 128,317] | [66,756; 127,012] | [65,872; 125,329] | [66,118; 125,799] | [65,774; 125,144] | [72,282; 137,525] | [68,847; 130,991] | |
| Medical costs averted (millions US$) | 173.8 | 15.3 | 80.5 | 7.5 | 207.7 | 20.5 | 199.8 | 505.3 |
| [104.8; 263.3] | [9.0; 23.5] | [48.1; 122.7] | [4.4; 11.6] | [122.8; 319.3] | [12.2; 31.5] | [120.9; 301.7] | [301.7; 771.6] | |
| Net costs (millions US$) | 5511 | 191 | 1847 | 184 | 5157 | 709 | 6635 | 13,599 |
| [4328; 9028] | [147; 321] | [1441; 3067] | [143; 305] | [4024; 8558] | [556; 1173] | [5213; 10,871] | [10,641; 22,453] | |
| ICER without stunting burden (2016 US$ / DALY) | 2726 | 5496 | 4027 | 22,266 | 9019 | 16,719 | 3621 | 4334 |
| [1839; 5367] | [3616; 11,202] | [2712; 8012] | [14,678; 45,193] | [5972; 18,218] | [11,274; 33,246] | [2446; 7130] | [2912; 8625] | |
| ICER with stunting burden (2016 US$ / DALY) | 2177 | 4777 | 3279 | 19,434 | 7549 | 14,540 | 2909 | 3508 |
| [1469; 4334] | [3140; 9797] | [2214; 6591] | [12,789; 39,598] | [4983; 15,387] | [9,811; 29,020] | [1964; 5788] | [2357; 7037] | |
NOTE: Though vaccinations occur annually from 2025 to 2034, impacts are projected over the first five years of the vaccinated child’s life. Thus, the last year included in impact estimates is 2039.
Total deaths and DALYS are the sum of ETEC burden attributed to diarrhea from ETEC infection (direct) and ETEC-induced deaths from other infectious diseases.
Children: children under 5 years of age.
Fully vaccinated children (FVC): Number of eligible children who received all three doses of the vaccine.
Table 4.
Estimated disease burden associated with Shigella infection and Shigella vaccination impact in children under 5 years of age in 79 countries, by region. Model estimates are projected from 2025 (year of introduction) to 2034. Results from uncertainty analysis are listed below model estimates. Upper and Lower represent 95% uncertainty intervals for each estimate.
| AFRO | AMRO | EMRO | EURO | SEARO | WPRO | GAVI-eligible | Global | |
|---|---|---|---|---|---|---|---|---|
| Number of countries | 38 | 6 | 11 | 7 | 9 | 8 | 48 | 79 |
| Shigella disease burden | ||||||||
| MSD episodes (millions) | 50.61 | 2.31 | 37.21 | 0.09 | 27.59 | 0.36 | 68.82 | 118.16 |
| [49.38; 51.85] | [2.24; 2.37] | [36.53; 37.90] | [0.08; 0.11] | [26.85; 28.33] | [0.31; 0.40] | [67.25; 70.42] | [115.39; 120.96] | |
| Shigella-induced stunting cases (millions) | 9.02 | 0.35 | 6.38 | 0.01 | 5.60 | 0.05 | 12.58 | 21.41 |
| [5.53; 12.54] | [0.21; 0.49] | [3.91; 8.86] | [0.01; 0.02] | [3.44; 7.78] | [0.03; 0.08] | [7.71; 17.50] | [13.12; 29.77] | |
| Total Shigella deaths* (1000 s) | 183.79 | 2.49 | 112.59 | 0.02 | 41.26 | 0.17 | 224.34 | 340.32 |
| [138.51; 238.18] | [1.87; 3.26] | [85.71; 144.32] | [0.01; 0.02] | [30.44; 54.88] | [0.13; 0.22] | [169.92; 289.29] | [256.54; 440.83] | |
| Total Shigella deaths*/100,000 children** | 9.72 | 3.88 | 17.83 | 0.02 | 2.46 | 0.08 | 10.49 | 7.47 |
| [7.32; 12.59] | [2.90; 5.06] | [13.58; 22.86] | [0.02; 0.03] | [1.82; 3.28] | [0.057; 0.097] | [7.94; 13.53] | [5.63; 9.68] | |
| Other Infectious disease deaths from Shigella-induced stunting as a percentage of total Shigella deaths | 23 | 15 | 20 | 16 | 19 | 16 | 22 | 21 |
| [15; 29] | [10; 20] | [13; 26] | [10; 21] | [12; 24] | [11; 21] | [14; 28] | [14; 27] | |
| Total DALYs* (1000 s) | 6,366.9 | 89.6 | 3,925.1 | 0.6 | 1,456.7 | 6.2 | 7,797.4 | 11,845.1 |
| [4,800.8; 8,251.8] | [67.2; 117.1] | [2,986.6; 5,032.0] | [0.4; 0.8] | [1,075.0; 1,937.5] | [4.7; 8.0] | [5,906.3; 10,049.4] | [8,928.9; 15,346.9] | |
| Total DALYS* /100,000 children** | 336.6 | 139.3 | 621.7 | 0.9 | 87.0 | 2.8 | 364.6 | 260.0 |
| [253.8; 436.2] | [104.5; 182.1] | [473.1; 797.0] | [0.6; 1.2] | [64.2; 115.7] | [2.1; 3.6] | [276.2; 469.9] | [196.0; 336.9] | |
| Outpatient treatment costs (millions US$) | 212.9 | 12.8 | 220.2 | 0.2 | 189.7 | 1.1 | 324.3 | 636.9 |
| [147.4; 279.8] | [8.9; 16.8] | [152.4; 289.3] | [0.2; 0.3] | [131.3; 249.3] | [0.7; 1.4] | [224.6; 426.2] | [441.0; 837.0] | |
| Inpatient treatment costs (millions US$) | 178.6 | 15.5 | 215.8 | 0.3 | 225.3 | 1.2 | 250.4 | 636.7 |
| [97.8; 277.7] | [8.5; 24.1] | [118.2; 335.6] | [0.2; 0.5] | [123.4; 350.3] | [0.7; 1.9] | [137.2; 389.4] | [348.7; 990.1] | |
| SHIGELLA vaccination IMPACT | ||||||||
| MSD episodes averted (millions) | 24.97 | 1.24 | 19.62 | 0.02 | 14.95 | 0.11 | 35.85 | 60.90 |
| [18.57; 31.45] | [0.92; 1.56] | [14.59; 24.71] | [0.01; 0.02] | [11.11; 18.82] | [0.08; 0.13] | [26.65; 45.15] | [45.28; 76.71] | |
| Shigella-induced stunting cases averted (millions) | 4.30 | 0.19 | 3.32 | 0.002 | 3.03 | 0.02 | 6.39 | 10.86 |
| [2.46; 6.56] | [0.11; 0.29] | [1.90; 5.06] | [0.001; 0.004] | [1.7; 4.6] | [0.01; 0.02] | [3.65; 9.75] | [6.20; 16.56] | |
| Direct diarrheal deaths averted (1000 s) | 64.84 | 1.04 | 42.44 | 0.007 | 18.16 | 0.08 | 85.37 | 126.57 |
| [43.27; 90.95] | [0.69; 1.48] | [28.40; 59.25] | [0.005; 0.011] | [11.93; 26.12] | [0.05; 0.11] | [57.03; 119.57] | [84.41; 177.84] | |
| Direct diarrheal deaths averted/100,000 FVC | 4.15 | 1.79 | 7.80 | 0.01 | 1.20 | 0.04 | 4.54 | 3.21 |
| [2.77; 5.82] | [1.19; 2.55] | [5.22; 10.89] | [0.01; 0.02] | [0.79; 1.72] | [0.02; 0.05] | [3.03; 6.35] | [2.14; 4.51] | |
| Infectious disease deaths due to induced stunting averted (1000 s) | 19.15 | 0.18 | 10.72 | 0.001 | 4.16 | 0.01 | 23.48 | 34.23 |
| [10.25; 31.07] | [0.10; 0.30] | [5.75; 17.31] | [0.001; 0.002] | [2.19; 6.87] | [0.01; 0.02] | [12.59; 38.01] | [18.31; 55.54] | |
| Infectious disease deaths due to induced stunting/100,000 FVC | 1.23 | 0.32 | 1.97 | 0.003 | 0.27 | 0.007 | 1.25 | 0.87 |
| [0.66; 1.99] | [0.17; 0.52] | [1.06; 3.18] | [0.001; 0.004] | [0.14; 0.45] | [0.004; 0.012] | [0.67; 2.02] | [0.46; 1.41] | |
| Total deaths averted* (1000 s) | 83.99 | 1.22 | 53.16 | 0.009 | 22.32 | 0.09 | 108.85 | 160.80 |
| [55.51; 119.06] | [0.81; 1.75] | [35.32; 74.90] | [0.006; 0.013] | [14.59; 32.28] | [0.06; 0.13] | [72.19; 154.03] | [106.38; 228.16] | |
| Total deaths averted*/100,000 FVC*** | 5.38 | 2.11 | 9.77 | 0.02 | 1.47 | 0.04 | 5.78 | 4.08 |
| [3.55; 7.62] | [1.39; 3.02] | [6.49; 13.76] | [0.01; 0.02] | [0.96; 2.13] | [0.029; 0.062] | [3.84; 8.18] | [2.70; 5.78] | |
| Total DALYs* averted (1000 s) | 2,779.5 | 41.1 | 1,782.4 | 0.3 | 744.4 | 3.0 | 3,625.1 | 5,350.7 |
| [1,843.0; 3,933.7] | [27.1; 58.7] | [1,186.1; 2,510.8] | [0.2; 0.4] | [487.1; 1,073.3] | [2.0; 4.3] | [2,408.5; 5,117.7] | [3,554.8; 7,580.2] | |
| Total DALYs* averted/100,000 FVC*** | 177.9 | 70.9 | 327.5 | 0.5 | 49.0 | 1.5 | 192.6 | 135.7 |
| [118.0; 251.8] | [46.8; 101.3] | [218.0; 461.4] | [0.4; 0.8] | [32.1; 70.7] | [1.0; 2.1] | [128.0; 271.9] | [90.1; 192.2] | |
| Vaccination costs (millions US$) | 5,685 | 206 | 1,928 | 191 | 5,365 | 730 | 6,835 | 14,104 |
| [4,509; 9,190] | [163; 335] | [1,523; 3,144] | [151; 313] | [4,239; 8,760] | [576; 1,192] | [5,421; 11,057] | [11,165; 22,933] | |
| Vaccination costs/100,000 FVC*** | 363,978 | 355,339 | 354,231 | 352,802 | 353,201 | 352,645 | 363,155 | 357,608 |
| [288,677; 588,335] | [280,891; 578,924] | [279,949; 577,793] | [278,730; 576,355] | [279,075; 576,739] | [278,583; 576,204] | [288,028; 587,469] | [283,072; 581,456] | |
| Administration costs/100,000 FVC*** | 105,908 | 98,125 | 97,127 | 95,840 | 96,199 | 95,698 | 105,166 | 100,169 |
| [73,377; 138,875] | [67,984; 128,669] | [67,293; 127,360] | [66,401; 125,672] | [66,650; 126,144] | [66,303; 125,487] | [72,863; 137,902] | [69,401; 131,350] | |
| Medical costs averted (millions US$) | 191.8 | 15.7 | 226.2 | 0.2 | 225.4 | 1.3 | 297.6 | 660.6 |
| [115.0; 288.0] | [9.2; 23.9] | [134.4; 341.4] | [0.1; 0.4] | [132.5; 342.1] | [0.7; 1.9] | [179.3; 446.3] | [392.2; 997.1] | |
| Net costs (millions US$) | 5,493 | 190 | 1,701 | 191 | 5,139 | 728 | 6,537 | 13,444 |
| [4,323; 9,000] | [147; 320] | [1,286; 2,922] | [151; 312] | [4,007; 8,535] | [575; 1,191] | [5,124; 10,759] | [10,496; 22,277] | |
| ICER without stunting burden (2016 US$ / DALY) | 2,485 | 5,339 | 1,175 | 746,611 | 8,282 | 276,909 | 2,245 | 3,114 |
| [1,700; 4,949] | [3,562; 11,042] | [779; 2,450] | [504,555; 1,505,798] | [5,561; 16,963] | [190,741; 549,697] | [1,532; 4,492] | [2,117; 6,276] | |
| ICER with stunting burden (2016 US$ / DALY) | 1,976 | 4,627 | 955 | 649,033 | 6,904 | 239,964 | 1,803 | 2,513 |
| [1,350; 3,950] | [3,093; 9,584] | [631; 1,999] | [441,268; 1,314,833] | [4,647; 14,221] | [165,492; 477,401] | [1,229; 3,621] | [1,708; 5,088] | |
Note: Though vaccinations occur annually from 2025 to 2034, impacts are projected over the first five years of the vaccinated child’s life. Thus, the last year included in impact estimates is 2039.
Total deaths and DALYS are the sum of Shigella burden attributed to diarrhea from Shigella infection (direct) and Shigella-induced deaths from other infectious diseases.
Children: children under 5 years of age.
Fully vaccinated children (FVC): Number of eligible children who received all three doses of the vaccine.
Introducing ETEC or Shigella vaccines would prevent 92,000 [61,200; 129,900] ETEC and 126,600 [84,300; 179,600] Shigella direct deaths and 21,800 [11.29; 34.75] ETEC- and 34,200 [18,000; 56,000] Shigella-induced stunting deaths from other infectious diseases over the first 10 years (Table 3, Table 4). ETEC and Shigella vaccination would prevent 17.7 [10.0; 27.0] million cases of moderate or severe stunting. ETEC vaccination would prevent 2.9 [1.9; 4.1] deaths/100,000 vaccinated children, globally, with the greatest benefit in AFRO at 4.7 [3.1; 6.7] deaths averted/100,000 vaccinated children. Shigella vaccination would prevent 4.0 [2.7; 5.9] deaths/100,000 vaccinated children, globally, with the highest reduction in EMRO at 9.8 [6.5; 13.9] deaths averted/100,000 vaccinated children. Vaccination would prevent 51% and 52% of the global ETEC and Shigella diarrheal episodes, respectively.
We also analyzed results for Gavi-eligible countries only. These 48 countries account for over 60% of the total ETEC and Shigella burden (Table 3, Table 4). ETEC and Shigella vaccines in these countries can prevent over 66,700 [44,100; 94,700] ETEC and 108,000 [72,200; 156,000] Shigella deaths. The vaccines are projected to avert 61.4 [44.6; 77.4] million cases of MSD and approximately 10 [6; 15] million cases of moderate-to-severe stunting over the first 5 years of life in GAVI-eligible LMICs.
3.2. Cost-effectiveness of ETEC and Shigella vaccination
Globally, the ICER for ETEC vaccination is estimated at $3508 [2357; 7037]/DALY averted and $2513 [1708; 5088]/DALY for Shigella vaccination from 2025 to 2034 (Table 3, Table 4). Regional ETEC ICERs range from $2177 [1469; 4334]/DALY in AFRO to $19,434 [12,789; 39,598]/DALY in EURO. Regional Shigella ICERs range from $955 [631; 1999]/DALY in EMRO to $649,033 [441,268; 1,314,833]/DALY in EURO.
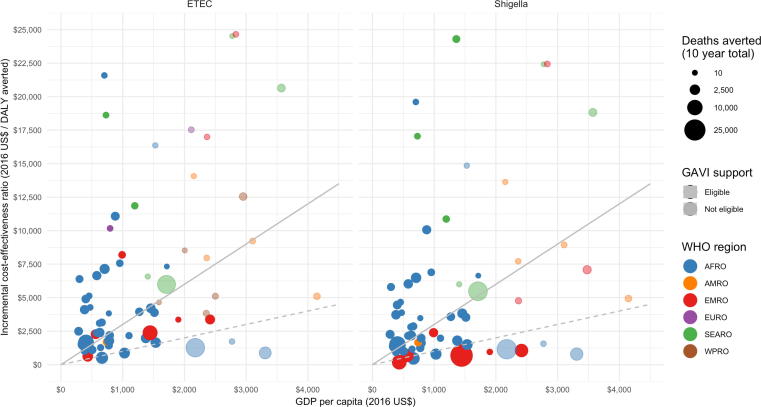
ETEC and Shigella vaccines met the ‘cost-effective’ threshold (ICER < 3XGDP) in 27 [10; 34] and 29 [14; 37] countries, respectively (Table 5), and ‘very cost-effective’ threshold (ICER < GDP) in 6 [1; 8] and 11 [3; 14] countries, respectively (Table 6). The majority of these countries are in AFRO (Fig. 1). When only countries with ‘cost-effective’ ICERs are considered, the global ETEC ICER is $1632 [1449; 2530]/DALY and Shigella is $1117 [1045; 1687]/DALY, lower than the global ICERs when all countries are considered. In countries meeting the ‘very cost-effective’ threshold, global ICERs are most favorable at $1061 [828; 1941]/DALY and $810 [540; 1413]/DALY for ETEC and Shigella vaccination, respectively. Implementing in the ‘cost-effective’ countries would achieve 53% and 66% of the potential benefit ETEC and Shigella vaccination, respectively, in all countries, at 25% and 30% of the net costs.
Table 5.
Cost-effectiveness of standalone vaccines for ETEC and Shigella, when limited to settings with an ICER less than three times the GDP threshold, by region, 2025–2034. Results from uncertainty analysis are listed below model estimates. Upper and Lower represent 95% uncertainty intervals for each estimate.
| AFRO | AMRO | EMRO | SEARO | WPRO** | GAVI-eligible | Global | |
|---|---|---|---|---|---|---|---|
| ETEC vaccination | |||||||
| Number of countries ICER < 3 X GDP | 17 | 3 | 4 | – | 3 | 19 | 27 |
| [7; 21] | [1; 4] | [2; 5] | – | [ND; 3] | [6; 24] | [10; 34] | |
| Total deaths* averted (1000 s) | 45.6 | 1.0 | 13.6 | – | 0.4 | 35.0 | 60.7 |
| [23.6; 71.3] | [0.2; 1.5] | [2.7; 19.4] | – | [ND; 0.6] | [10.0; 59.3] | [26.8; 116.1] | |
| Total deaths* averted/100,000 FVC*** | 7.2 | 2.5 | 4.6 | – | 2.2 | 5.4 | 6.1 |
| [5.5; 9.9] | [1.2; 3.7] | [2.8; 6.2] | – | [ND; 3.0] | [4.1; 7.6] | [3.9; 8.0] | |
| Total DALYs* averted (1000 s) | 1,561 | 35 | 464 | – | 13 | 1,194 | 2,073 |
| [807; 2,440] | [8; 51] | [93; 659] | – | [ND; 19] | [341; 2,026] | [915; 3,970] | |
| Total DALYs* averted/100,000 FVC*** | 246 | 86 | 155 | – | 76 | 185 | 209 |
| [190; 340] | [40; 128] | [94; 211] | – | [ND; 100] | [140; 260] | [133; 273] | |
| Vaccination costs (millions US$) | 2,259 | 146 | 1,055 | – | 60 | 2,295 | 3,520 |
| [1,550; 2,944] | [69; 183] | [332; 1,631] | – | [ND; 95] | [821; 3,026] | [2,333; 7,928] | |
| Vaccination costs/100,000 FVC*** | 355,702 | 356,373 | 353,238 | – | 352,397 | 356,570 | 354,931 |
| [274,110; 561,392] | [265,267; 557,867] | [253,764; 557,408] | – | [ND; 546,333] | [274,842; 563,324] | [272,039; 561,229] | |
| Administration costs/100,000 FVC*** | 98,453 | 99,057 | 96,233 | – | 95,475 | 99,234 | 97,757 |
| [65,886; 125,759] | [63,968; 126,238] | [60,760; 123,783] | – | [ND; 122,304] | [66,462; 127,364] | [65,076; 124,893] | |
| Medical costs averted (millions US$) | 82 | 10 | 43 | – | 2 | 80 | 137 |
| [34; 127] | [3; 17] | [7; 62] | – | [ND; 4] | [13; 125] | [49; 374] | |
| ICER (2016 US$/DALY) | 1,395 | 3,871 | 2,181 | – | 4,474 | 1,856 | 1,632 |
| [982; 2,154] | [2,479; 9,664] | [1,203; 3,681] | – | [ND; 6,655] | [1,206; 2,762] | [1,449; 2,530] | |
| Shigella vaccination | |||||||
| Number of countries ICER < 3 X GDP | 18 | 3 | 8 | – | – | 22 | 29 |
| [8; 23] | [1; 4] | [5; 8] | – | – | [10; 27] | [14; 37] | |
| Total deaths* averted (1000 s) | 52.7 | 1.1 | 53.0 | – | – | 76.5 | 106.8 |
| [27.1; 91.0] | [0.2; 1.6] | [31.9; 71.9] | – | – | [41.1; 121.4] | [59.4; 188.3] | |
| Total deaths* averted/100,000 FVC*** | 7.9 | 2.6 | 10.7 | – | – | 10.1 | 8.8 |
| [6.1; 10.9] | [1.2; 3.9] | [8.1; 15.2] | – | – | [8.2; 14.2] | [5.3; 11.9] | |
| Total DALYs* averted (1000 s) | 1,746 | 36 | 1,777 | – | – | 2,556 | 3,559 |
| [901; 3,010] | [8; 53] | [1,070; 2,412] | – | – | [1,377; 4,041] | [1,983; 6,255] | |
| Total DALYs* averted/100,000 FVC*** | 260 | 89 | 357 | – | – | 339 | 294 |
| [202; 361] | [41; 130] | [270; 508] | – | – | [273; 476] | [176; 395] | |
| Vaccination costs (millions US$) | 2,386 | 146 | 1,763 | – | – | 2,693 | 4,296 |
| [1,650; 3,237] | [71; 187] | [1,250; 2,426] | – | – | [1,811; 3,597] | [3,030; 9,202] | |
| Vaccination costs/100,000 FVC*** | 355,503 | 356,373 | 354,360 | – | – | 356,897 | 355,062 |
| [275,835; 560,639] | [268,790; 558,302] | [272,641; 560,870] | – | – | [276,720; 562,627] | [273,263; 560,780] | |
| Administration costs/100,000 FVC*** | 98,273 | 99,057 | 97,243 | – | – | 99,529 | 97,876 |
| [66,166; 125,794] | [64,733; 125,497] | [65,110; 124,785] | – | – | [67,012; 127,662] | [65,318; 124,896] | |
| Medical costs averted (millions US$) | 98 | 10 | 213 | – | – | 184 | 321 |
| [38; 151] | [3; 17] | [74; 302] | – | – | [81; 269] | [116; 652] | |
| ICER (2016 US$/DALY) | 1,311 | 3,750 | 872 | – | – | 981 | 1,117 |
| [899; 1,960] | [2,465; 9,416] | [539; 1,260] | – | – | [713; 1,366] | [1,045; 1,687] | |
Total deaths and DALYS are the sum of burden attributed to diarrhea from ETEC or Shigella infection and ETEC- or Shigella-induced deaths from other infectious diseases.
“ND” indicates ‘not defined’ as there were no WPRO countries that met the threshold under the predicted lower bounds (2.5%) of the 95% uncertainty intervals.
Fully vaccinated children (FVC): Number of eligible children who received all three doses of the vaccine.
Table 6.
Cost-effectiveness of standalone vaccines for ETEC and Shigella, when limited to settings with an ICER less than GDP threshold, by region, 2025–2034. Results from uncertainty analysis are listed below model estimates. Upper and Lower represent 95% uncertainty intervals for each estimate.
| AFRO | AMRO | EMRO | SEARO | WPRO | GAVI-eligible** | Global | |
|---|---|---|---|---|---|---|---|
| ETEC vaccination | |||||||
| Number of countries ICER < GDP | 6 | – | – | – | – | 3 | 6 |
| [1; 7] | – | – | – | – | [ND; 4] | [1; 8] | |
| Total deaths* averted (1000 s) | 32 | – | – | – | – | 8 | 32 |
| [3; 46] | – | – | – | – | [ND; 14] | [3; 48] | |
| Total deaths* averted/100,000 FVC** | 9 | – | – | – | – | 16 | 5 |
| [6; 12] | – | – | – | – | [ND; 22] | [1; 7] | |
| Total DALYs* averted (1000 s) | 1108.1 | – | – | – | – | 259.4 | 1108.1 |
| [119; 1590] | – | – | – | – | [ND; 477] | [119; 1645] | |
| Total DALYs* averted/100,000 FVC** | 320 | – | – | – | – | 548 | 320 |
| [196; 416] | – | – | – | – | [ND; 756] | [196; 407] | |
| Vaccination costs (millions US$) | 1226 | – | – | – | – | 175 | 1226 |
| [229; 1690] | – | – | – | – | [ND; 387] | [229; 1732] | |
| Vaccination costs/100,000 FVC*** | 354,236 | – | – | – | – | 369,582 | 354,236 |
| [272,778; 559,003] | – | – | – | – | [ND; 500,700] | [272,788; 559,003] | |
| Administration costs/100,000 FVC*** | 97,132 | – | – | – | – | 110,957 | 97,132 |
| [64,858; 122,847] | – | – | – | – | [ND; 136,183] | [64,853; 122,847] | |
| Medical costs averted (millions US$) | 50 | – | – | – | – | 4 | 50 |
| [14; 75] | – | – | – | – | [ND; 13] | [14; 81] | |
| ICER (2016 US$/DALY) | 1,061 | – | – | – | – | 660 | 1,061 |
| [746; 1,941] | – | – | – | – | [ND; 845] | [828; 1,941] | |
| Shigella vaccination | |||||||
| Number of countries ICER < GDP | 7 | – | 4 | – | – | 8 | 11 |
| [1; 8] | – | [2; 5] | – | – | [2; 10] | [3; 14] | |
| Total deaths* averted (1000 s) | 40 | – | 44 | – | – | 55 | 84 |
| [4; 55] | – | [11; 65] | – | – | [11; 82] | [15; 120] | |
| Total deaths* averted/100,000 FVC** | 10 | – | 15 | – | – | 14 | 12 |
| [6; 14] | – | [10; 20] | – | – | [10; 19] | [8; 16] | |
| Total DALYs* averted (1000 s) | 1312 | – | 1476 | – | – | 1856 | 2787 |
| [138; 1806] | – | [365; 2181] | – | – | [365; 2,727] | [510; 3,996] | |
| Total DALYs* averted/100,000 FVC** | 338 | – | 494 | – | – | 478 | 406 |
| [213; 453] | – | [320; 666] | – | – | [323; 647] | [267; 542] | |
| Vaccination costs (millions US$) | 1,375 | – | 1,055 | – | – | 1,379 | 2,430 |
| [256; 1,759] | – | [442; 1,644] | – | – | [442; 1,778] | [713; 3,353] | |
| Vaccination costs/100,000 FVC*** | 354,016 | – | 353,238 | – | – | 355,120 | 353,678 |
| [271,616; 556,691] | – | [264,699; 556,090] | – | – | [266,212; 556,324] | [271,471; 557,353] | |
| Administration costs/100,000 FVC*** | 96,933 | – | 96,233 | – | – | 97,928 | 96,629 |
| [64,380; 123,469] | – | [63,434; 123,544] | – | – | [64,354; 125,143] | [64,302; 123,565] | |
| Medical costs averted (millions US$) | 60 | – | 113 | – | – | 123 | 173 |
| [16; 85] | – | [13; 168] | – | – | [13; 178] | [31; 256] | |
| ICER (2016 US$/DALY) | 1,002 | – | 638 | – | – | 677 | 810 |
| [674; 1,865] | – | [402; 1,187] | – | – | [433; 1,187] | [540; 1,413] | |
Total deaths and DALYS are the sum of burden attributed to diarrhea from ETEC or Shigella infection and ETEC- or Shigella-induced deaths from other infectious diseases.
“ND” indicates ‘not defined’ as there were no GAVI-eligible countries that met the threshold under the predicted lower bounds (2.5%) of the 95% uncertainty intervals.
Fully vaccinated children (FVC): Number of eligible children who received all three doses of the vaccine
Fig. 1.
Incremental cost-effectiveness of ETEC and Shigella standalone vaccines by national Gross Domestic Product (2025–2034). The solid line represents a threshold of ICERs relative to values of three times the GDP, while the dashed line represents ICERS relative to GDP. Countries following below either line would be considered cost-effective based on each criterion.
ICERs for ETEC and Shigella vaccines were more favorable at $2909 [1964; 5788]/DALY and $1803 [1229; 3621]/DALY when only considering the 48 GAVI-eligible countries (Table 3, Table 4). In GAVI-eligible countries where vaccines were considered ‘cost-effective’ (ICER < 3XGDP, ETEC: 19 [6; 24], Shigella; 22 [10; 27] countries), ICERs were $1856 [1206; 2762]/DALY and $981 [713; 1366]/DALY for ETEC and Shigella vaccines, respectively (Table 5). ICERs improved further to $660 [not defined; 845]/DALY (ETEC) and $677 [433; 1187]/DALY (Shigella) when including only ‘very cost-effective’ (ICER < GDP, ETEC: four countries, Shigella; nine countries) GAVI-eligible countries (Table 6).
3.3. Sensitivity and uncertainty
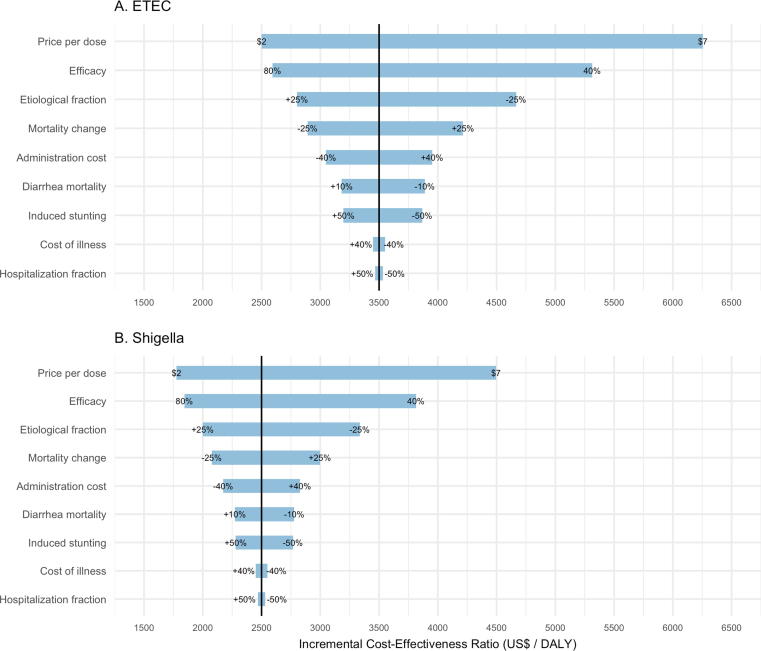
Globally, the most influential variables on ICER estimates were vaccine price per dose (range; $3765/DALY [ETEC] and $2727/DALY [Shigella]) and efficacy (range; $2729/DALY [ETEC] and $1977/DALY [Shigella]), followed by the etiological fraction attributed to each pathogen (range; $1871/DALY [ETEC] and $1340/DALY [Shigella]) (Fig. 2A and B). Variation in projected mortality change (range; $1326/DALY [ETEC] and $923/DALY [Shigella]) was also influential on ICERs.
Fig. 2.
Tornado diagram showing results of one-way PSA exploring the affect key input variables have on cost-effectiveness of ETEC and Shigella standalone vaccines, in 79 LMICs from 2025 to 2034. Ranges of variables (listed in Table 2) are displayed at the end of the corresponding bar. Price per dose is varied by $US ranging from a low estimate of $2/dose to a high estimate of $7/dose. ‘Etiological fraction’ is variation in the fraction of overall diarrheal mortality attributed to ETEC and Shigella diarrhea. ‘Mortality change’ is variation in the rates diarrheal mortality projected in years 2025–2034. ‘Induced stunting’ refers to the number of other infectious disease deaths caused by ETEC or Shigella induced stunting. ‘Hospitalized fraction’ is variation in the fraction of children hospitalized (1 in 8 referred to inpatient facility).
4. Discussion
Our analysis is the first evaluating the impact and cost-effectiveness of potential ETEC and Shigella vaccine candidates in children in low and lower-middle income countries. The impact of vaccination on stunting and deaths from other infectious diseases makes ETEC and Shigella vaccination more compelling and cost-effective. These vaccines could avert over 274,000 ETEC and Shigella attributable deaths in the first decade, a 47% reduction in mortality. Including benefits of stunting averted results in much lower ICERs and increases the number of countries where vaccination is cost-effective.
Our results suggest that there is heterogeneity in vaccine impact and cost-effectiveness across regions and by Gavi eligibility. While the majority of the averted burden for both ETEC and Shigella vaccines is in AFRO, substantial burden is also averted in EMRO. Introducing vaccines only in high burden countries and/regions could reduce cost without substantial reductions in health impact. This is clearest when calculating impact for those countries where vaccines are cost-effective. The number of deaths averted per 100,000 fully vaccinated children for an ETEC vaccine increases, while still averting the majority of preventable total ETEC deaths. Gavi-eligible countries may also benefit greatly as these countries experience a large share of projected disease burden. Vaccine introduction in these countries alone could have a substantial impact on burden. It will be important for country policy makers to understand disease heterogeneity when evaluating whether or not to introduce these vaccines.
There is evidence that childhood stunting may be associated with chronic diseases such as heightened prevalence of high blood pressure, impaired fasting glucose, and increased body mass index [9]. If ETEC and Shigella infections increase chronic disease through induced stunting, vaccination could be even more impactful and cost-effective than initially realized. In addition to chronic disease risk, early childhood stunting has been shown to be associated with decreased earnings and fewer completed years of school [51]. If ETEC and Shigella vaccination could reduce stunting and these long-term health and development consequences, then vaccination is more economically viable than indicated in the results from this study.
The model incorporates projections based on population forecasts and past diarrheal mortality trends that could contribute to underestimation of vaccine impacts. First, while the past two decades have seen consistent declines in diarrheal mortality [52], additional factors may alter this trend. Global urbanization rates are increasing in low- and lower-middle income countries, placing larger populations at risk as growth overwhelms infrastructure, forcing many into living in underserved informal settlements. Risk for infectious diseases increase in these environments due to overcrowding with lack of safe sanitation and clean water [53]. While moving to urban areas could increase access to health care and therefore reduce mortality, this is highly dependent on individual household economic status and their community’s urban infrastructure which is highly variable within and between cities. There is some evidence that a move to urban areas reduces access to health care [54]. Second, increasing antibiotic resistance and climate change may reverse gains made over the past few years by increasing risk of exposure, severe disease, and death. Third, vaccination impact could be underestimated if these vaccines induce herd protection. Fourth, dmLT adjuvant is in current formulation which has potential to improve protection. Evidence of mucosal immune responses induced by ETVAX vaccine may provide some protection against ETEC colonization factor antigens not included in the vaccine [55].
There are several limitations to this analysis. First, there are no vaccine trial-derived measures of vaccine efficacy and duration. As these vaccine candidates are under development, we do not know their price, thus our cost-effectiveness estimates may be higher or lower than projected. Furthermore, our cost-effectiveness estimates are dependent on assuming that these two vaccines would have the same price, efficacy and coverage.
No treatment-seeking or hospitalization rates exist for children experiencing ETEC and Shigella episodes in most of our study countries. We used DHS data on treatment seeking for diarrhea and assumed a proportion of those children are hospitalized. We also adjusted our fraction of MSD episodes based on GEMS methodology. Thus, we have assumed that all MSD cases access treatment which may underestimate the true number of child MSD. We modelled cost of illness based on available estimates—this approach may over- or under-estimate the medical cost averted due to vaccination in many countries. For most input parameters, we have limited information on the true degree of uncertainty and thus we rely on simple distribution types and measures of dispersion. We did not estimate waning in our model or the impact of partial protection from receiving less than three doses.
A final limitation is the rate of decline for diarrheal mortality. We assumed mortality decline mirrors the decline seen during 2000–2013. However, studies are actively evaluating whether the rate of decline is decreasing. Incorporating these findings may alter our estimates of vaccine impact by increasing the number of deaths averted.
This analysis considered the potential impact and cost-effectiveness of vaccines that are in development. It shows the importance of including the expanded impact of vaccination to prevent stunting and resulting deaths due to other infectious disease. To capture the true value of these vaccines, it is important to quantify the expanded effects of enteric pathogens. Burden was concentrated in a few regions, indicating that introduction in these countries could potentially avert the majority of disease burden globally. As these vaccines undergo further clinical development, it will be important to reassess the potential vaccine impact as data on efficacy, duration of protection, and diarrheal impact on stunting becomes available.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgement
This work was funded by a grant to PATH from the Bill & Melinda Gates Foundation (OPP1112376).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2019.100024.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Kotloff K.L., Platts-Mills J.A., Nasrin D., Roose A., Blackwelder W.C., Levine M.M. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine. 2017;35:6783–6789. doi: 10.1016/j.vaccine.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Colombara D.V., Khalil I.A.-M., Rao P.C., Troeger C., Forouzanfar M.H., Riddle M.S. Chronic health consequences of acute enteric infections in the developing. World Am J Gastroenterol Suppl. 2016;3:4–11. [Google Scholar]
- 6.Anderson J.D., Bagamian K.H., Muhib F., Amaya M.P., Laytner L.A., Wierzba T. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Checkley W., Buckley G., Gilman R.H., Assis A.M., Guerrant R.L., Morris S.S. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey J.H. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 9.Guerrant R.L., DeBoer M.D., Moore S.R., Scharf R.J., Lima A.A.M. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2012;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371 doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 11.Kotloff K.L., Blackwelder W.C., Nasrin D., Nataro J.P., Farag T.H., van Eijk A. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55:S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosangadi D., Smith P.G., Giersing B.K. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atherly D.E., Lewis K.D.C., Tate J., Parashar U.D., Rheingans R.D. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011–2030. Vaccine. 2012;30:A7–A14. doi: 10.1016/j.vaccine.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 14.Rheingans R., Atherly D., Anderson J. Distributional impact of rotavirus vaccination in 25 GAVI countries: Estimating disparities in benefits and cost-effectiveness. Vaccine. 2012;30(Supplement 1):A15–A23. doi: 10.1016/j.vaccine.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 15.United Nations. World population prospects: the 2015 revision, key findings and advance tables. United Nations, Department of Economic and Social Affairs, Population Division; 2015.
- 16.Lamberti L.M., Bourgeois A.L., Fischer Walker C.L., Black R.E., Sack D. Estimating Diarrheal illness and deaths attributable to shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Gratz J., Amour C., Kibiki G., Becker S., Janaki L. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Platts-Mills J.A., Juma J., Kabir F., Nkeze J., Okoi C. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. The Lancet. 2016;388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 2015; 385: p. 117–71. 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed]
- 20.World Health Organization. Global Health Observatory data repository. World Health Organ 2017. http://apps.who.int/gho/data/node.home [accessed 01.02.17].
- 21.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, Network for the WHO-CGRS, Agocs M, et al. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis 2016; 62: S96–105. 10.1093/cid/civ1013. [DOI] [PubMed]
- 22.Lanata C.F., Fischer Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE. 2013;8:e72788–e72811. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute for Health Metrics and Evaluation (IHME). GBD Compare Viz Hub 2016. http://vizhub.healthdata.org/gbd-compare/.
- 24.USAID. Demographic and Health Survey (DHS) STATcompiler n.d. http://www.statcompiler.com/en/ [accessed 06.12.16].
- 25.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prüss-Üstün A., Corvalán C. World Health Organization; Geneva, Switzerland: 2006. Preventing disease through healthy environments: towards an estimate of the environmental burden of disease. [Google Scholar]
- 27.Murray CJL, Lopez AD, Organization WH, Bank W, Health HS of P. The Global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary 1996.
- 28.WHO, UNICEF. WHO and UNICEF estimates of national infant immunization coverage; 2017. [DOI] [PMC free article] [PubMed]
- 29.GAVI The Vaccine Alliance. Detailed Product Profiles (DPPs) for WHO prequalified vaccines; 2018.
- 30.Parashar U.D., Hummelman E.G., Bresee J.S., Miller M.A., Glass R.I. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Single Drug Information | International Medical Products Price Guide n.d. http://mshpriceguide.org/en/single-drug-information/ [accessed 22.12.17].
- 32.Hendrix N., Bar-Zeev N., Atherly D., Chikafa J., Mvula H., Wachepa R. The economic impact of childhood acute gastroenteritis on Malawian families and the healthcare system. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rheingans R., Kukla M., Faruque A.S.G., Sur D., Zaidi A.K.M., Nasrin D. Determinants of household costs associated with childhood diarrhea in 3 South Asian settings. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55(Suppl 4):S327–S335. doi: 10.1093/cid/cis764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rheingans R., Kukla M., Adegbola R.A., Saha D., Omore R., Breiman R.F. Exploring household economic impacts of childhood diarrheal illnesses in 3 African settings. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55(Suppl 4):S317–S326. doi: 10.1093/cid/cis763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilopo S.A., Kilgore P., Kosen S., Soenarto Y., Aminah S., Cahyono A. Economic evaluation of a routine rotavirus vaccination programme in Indonesia. Vaccine. 2009;27(Suppl 5):F67–F74. doi: 10.1016/j.vaccine.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 36.Riewpaiboon A., Shin S., Le T.P.M., Vu D.T., Nguyen T.H.A., Alexander N. Cost of rotavirus diarrhea for programmatic evaluation of vaccination in Vietnam. BMC Public Health. 2016;16:777. doi: 10.1186/s12889-016-3458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flem E.T., Latipov R., Nurmatov Z.S., Xue Y., Kasymbekova K.T., Rheingans R.D. Costs of diarrheal disease and the cost-effectiveness of a rotavirus vaccination program in kyrgyzstan. J Infect Dis. 2009;200(Suppl 1):S195–S202. doi: 10.1086/605040. [DOI] [PubMed] [Google Scholar]
- 38.Ngabo F., Mvundura M., Gazley L., Gatera M., Rugambwa C., Kayonga E. The economic burden attributable to a child’s inpatient admission for diarrheal disease in Rwanda. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0149805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osano B.O., Wang’ombe J.K., Kamenwa R.W., Wamalwa D. Cost analysis of care for children admitted to kenyatta national hospital with rotavirus gastroenteritis. Vaccine. 2011;29:4019–4024. doi: 10.1016/j.vaccine.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Sowmyanarayanan T.V., Patel T., Sarkar R., Broor S., Chitambar S.D., Krishnan T. Direct costs of hospitalization for rotavirus gastroenteritis in different health facilities in India. Indian J Med Res. 2012;136:68–73. [PMC free article] [PubMed] [Google Scholar]
- 41.Patel R.B., Stoklosa H., Shitole S., Shitole T., Sawant K., Nanarkar M. The high cost of diarrhoeal illness for urban slum households-a cost-recovery approach: a cohort study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke R.M., Smith E.R., Dahl R.M., Rebolledo P.A., Calderón M.del C., Cañipa B. The economic burden of pediatric gastroenteritis to Bolivian families: a cross-sectional study of correlates of catastrophic cost and overall cost burden. BMC Public Health. 2014;14:642. doi: 10.1186/1471-2458-14-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke R.M., Rebolledo P.A., Embrey S.R., Wagner L.D., Cowden C.L., Kelly F.M. The burden of pediatric diarrhea: a cross-sectional study of incurred costs and perceptions of cost among Bolivian families. BMC Public Health. 2013;13:708. doi: 10.1186/1471-2458-13-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang V.M., Tran T.A., Ha A.D., Nguyen V.H. Cost of hospitalization for foodborne diarrhea: a case study from Vietnam. J Korean Med Sci. 2015;30(Suppl 2):S178–S182. doi: 10.3346/jkms.2015.30.S2.S178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew A., Srinivasan R., Venugopal S., Kang G. Direct medical costs in children with rotavirus and non-rotavirus diarrhea admitted to a pediatric intensive care unit and high dependency unit in Delhi. Indian Pediatr. 2016;53:639–641. doi: 10.1007/s13312-016-0902-4. [DOI] [PubMed] [Google Scholar]
- 46.Jacob J., Joseph T.K., Srinivasan R., Kompithra R.Z., Simon A., Kang G. Direct and indirect costs of pediatric gastroenteritis in Vellore, India. Indian Pediatr. 2016;53:642–644. doi: 10.1007/s13312-016-0903-3. [DOI] [PubMed] [Google Scholar]
- 47.Halder A.K., Luby S.P., Akhter S., Ghosh P.K., Johnston R.B., Unicomb L. Incidences and costs of illness for diarrhea and acute respiratory infections for children <5 years of age in rural Bangladesh. Am J Trop Med Hyg. 2017;96:953–960. doi: 10.4269/ajtmh.16-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portnoy A., Ozawa S., Grewal S., Norman B.A., Rajgopal J., Gorham K.M. Costs of vaccine programs across 94 low- and middle-income countries. Vaccine. 2015;33:A99–A108. doi: 10.1016/j.vaccine.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 49.Hutubessy R., Chisholm D. Edejer TT-T. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1:8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Middleton M. TreePlan Software; San Fransisco, CA: 2016. SimVoi: The Monte Carlo Simulation Add-in for Mac Excell 2011–2016. [Google Scholar]
- 51.Dewey K.G., Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7:5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Reiner R.C. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hathi P., Haque S., Pant L., Coffey D., Spears D. Place and child health: the interaction of population density and sanitation in developing countries. Demography. 2017;54:337–360. doi: 10.1007/s13524-016-0538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shetty P. Health care for urban poor falls through the gap. The Lancet. 2011;377:627–628. [Google Scholar]
- 55.Leach S., Lundgren A., Carlin N., Löfstrand M., Svennerholm A.-M. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent enterotoxigenicEscherichia coli (ETEC) vaccine ETVAX. Vaccine. 2017;35:3966–3973. doi: 10.1016/j.vaccine.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Walker C.L.F., Black R.E. Diarrhoea morbidity and mortality in older children, adolescents, and adults. Epidemiol Infect. 2010;138:1215–1226. doi: 10.1017/S0950268810000592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.