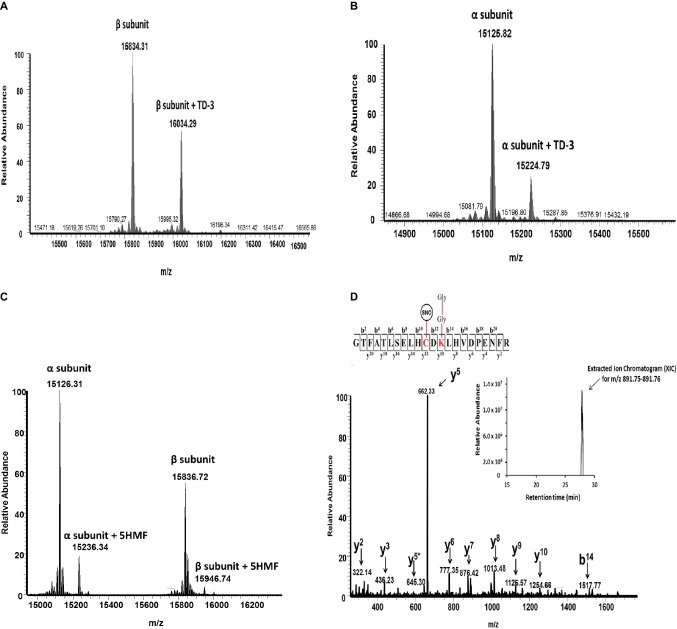
Figure 5.
Representative mass spectrometry data confirming the modification of HbS by TD-3, 5HMF, and HU. Intact mass (top-down) and LC/MS/MS (bottom-up) analysis were utilized to elucidate how the antisickling reagents interact with HbS. (A and B) Intact mass data confirm that TD-3 substantially modifies both β and α subunits. (C) Intact mass data confirms that 5HMF modifies both α and β subunits. (D) MS/MS fragmentation spectrum representing the y and b ions matched to the modified tryptic peptide GTFATSELHCDKLHVDPENFR (residues 83–104) from HbS incubated with HU. This peptide contains the diglycine signature modification (at βK96) associated with ubiquitination and S-nitrosylation (at βC93). The inset represents the extracted ion chromatogram representing the S-nitrosylated peptide.