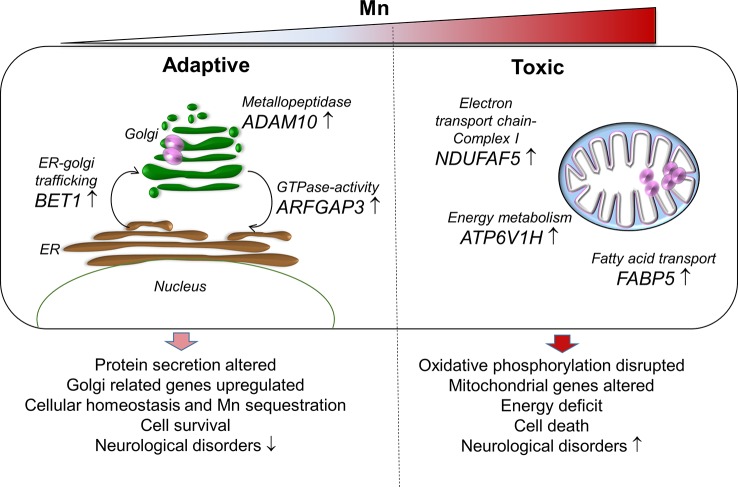
Figure 7.
A proposed schematic representation of Mn-dependent adaptive and toxic response pathways. Adaptive (left), human neuronal cells (SH-SY5Y) exposed to physiological Mn resulted in enrichment of protein secretion pathway by increasing gene expression for Golgi-residing proteins—BET1, ADAM10, and ARFGAP3. BET1 participates in vesicular transport from the endoplasmic reticulum (ER) to the Golgi complex thereby participating in protein transport and processing. ADAM10 has α-secretase activity in the neurons for proteolytic processing of the amyloid precursor proteins, thereby preventing accumulation of unwanted proteins. ARFGAP3 is a GTPase-activating protein that regulates the early secretory pathway of proteins. The protein secretion pathway plays an important role in Mn sequestration and maintaining cellular homeostasis, thus promoting cell survival that could protect against neurological diseases. Toxic (right), cells exposed to toxic Mn disrupts energy metabolism by enrichment in oxidative phosphorylation pathway involving genes that participate in energy metabolism—ATP6V1H, NDUFAF5, and FABP5. ATP6V1H is a gene in the oxidative phosphorylation pathway that couples ATPase activity to proton flow and mediates acidification of intracellular organelles. NDUFAF5 is a gene that is extremely essential in complex I assembly, which is required for the function of the mitochondrial electron transport chain. FABP5 is gene that regulates lipid metabolism by binding to long-chain fatty acids specifically and transporting them. Disruption in cellular and mitochondrial energy metabolism along with oxidative stress could lead to impending cell death and inability to recover. This combination could eventually be detrimental and result in Mn toxicity and neurological diseases.