Abstract
The aim of precision medicine is setting up targeted therapies for selected patients that would ideally have high effectiveness and few side effects. This is made possible by targeted therapy drugs that selectively act on a specific pathway. Precision medicine is spreading to many medical specialties, and there is increasing interest in the context of allergic airway diseases, such as allergic rhinitis, chronic rhinosinusitis, and asthma. This review is an update of new targets in the treatment of childhood allergic upper airway diseases and asthma, including the most recent biologic drugs that have already been licensed or are in the pipeline to be tested with children.
Keywords: allergic airway disease, allergic rhinitis, asthma, biologics, children, chronic rhinosinusitis, immunotherapy
Introduction
In the last decade, a large amount of research has provided consistent advances in molecular biology and ‘omics’ science (genomics, metabolomics, proteomics, and transcriptomics), which has led to the detailed characterization of the etiology, pathophysiological mechanisms, and subtypes of many diseases. Based on these developments, the concept of precision medicine was established. The aim of precision medicine is setting up targeted therapies, which would ideally have high effectiveness and few side effects, for selected patients. This is made possible because targeted therapy drugs selectively act on a specific pathway, corresponding to a particular endotype of the disease.1 Originally applied in oncology, precision medicine is spreading to other specialties, and there is increasing interest in the context of allergic airway disease (AAD). AADs include allergic rhinitis (AR), chronic rhinosinusitis (CRS), and asthma and show high prevalence in children.2
The management of severe forms of AAD is a priority and a challenge for clinicians. Asthma represents the prototype of AAD, and its management should be focused on controlling symptoms, improving quality of life, and preventing asthma attacks. To achieve this, a multidisciplinary approach is needed, along with the assessment of asthma control at regular intervals and better ways to detect airway remodeling. The evolution of disease management may also be on the control of remodeling in addition to symptom control, by means of newer therapies that may affect remodeling, preventing a decline in lung function. Based on periodic evaluations, therapy is stepped-down or stepped-up until the symptoms are under control. Nevertheless, a subgroup of patients remains uncontrolled, which include patients with difficult-to-treat asthma and severe asthma. The former is related to comorbidities, poor adherence to treatment, or exposure to environmental factors, while the latter includes therapy-resistant asthma.3,4 Patients with severe asthma are at high risk of developing acute severe exacerbations, also known as ‘status asthmaticus,’ a life-threatening condition unresponsive to repeated courses of β-agonist therapy.5
When the diagnosis of asthma is confirmed and comorbidities have been assessed, the European Respiratory Society and American Thoracic Society (ERS/ATS) guidelines define severe asthma as asthma that requires treatment with a high dose of inhaled corticosteroids (ICS) plus a second controller, asthma requiring systemic corticosteroids to prevent it from becoming ‘uncontrolled,’ or asthma that remains ‘uncontrolled’ despite this therapy.6 The aim of this review is to provide an update on novel therapeutic targets for AAD in children.
Methods
This review was conducted using two databases: PubMed and ScienceDirect. On these websites, we searched for articles in English using the following key words: allergic airway disease, allergy in children, asthma in children, phenotypes and endotypes of asthma, biomarkers in AAD, allergen immunotherapy (AIT), and novel biologics. As a rule of thumb, we preliminarily selected articles that we judged relevant on the basis of title. Then, we decided to use the abstracts of articles to assess whether they fit the topic. We found 1364 abstracts suitable for the topic. Among these, 208 were selected for detailed review. We also reviewed the references of the selected articles and read those with titles that might be of interest for the topic.
United airway disease as a model of diagnosis and care in severe AAD
In the context of AAD, research has shown a close link between upper and lower airway diseases. Their interplay is supported by epidemiologic, anatomical, histological, pathophysiologic, and therapeutic data. A high prevalence of AR (30–80%) has been reported among patients affected by asthma. The histological features of the upper and lower airways are similar and include a pseudostratified epithelium. They generally show an inflammatory pattern that is predominantly mediated by immunoglobulin-E (IgE).7 Hence, the term ‘allergic rhinobronchitis’ was first proposed.8
Over the last two decades, a shared pathophysiological mechanism has been hypothesized for AAD, where the bone marrow plays a key role in the systemic inflammatory response after an inflammatory response following the triggering of a specific site of the airways. The released mediators, especially interleukin (IL)-5, might reach the bone marrow and stimulate the mobilization of CD34+ cells and their differentiation into eosinophils, as well as blood eosinophil activation and increased IL-5 production, which lead to systemic inflammation and infiltration in different areas of the airways.9–10 Hallstrand and colleagues reported that nonallergic individuals who underwent bone marrow transplantation from donors with allergic disease had a high rate of developing AR and asthma during a long-term follow-up.11 Therefore, it may be appropriate to define AR, CRS, and asthma as different expressions of the same disease. Hence, the term United Airway Disease has been defined (Figure 1).12
Figure 1.
United airway disease model.
Many surveys, longitudinal studies, and retrospective studies have confirmed the association between AR and asthma.13–17 In 8 years of follow-up, 33% of individuals with AR developed bronchial hyperreactivity to methacholine bronchial challenge and impaired forced expiratory flow at 25–75% of the pulmonary volume (FEF25–75), compared to the control group, which showed no spirometric alteration.18 Reduced FEF25–75 in subjects with AR and in all subjects with normal forced expiratory value in 1 second (FEV1) may be an early marker of small airway impairment. FEF25–75 reduction is positively correlated with the severity of allergic predictors.19
In a review by Sedaghat, AR also emerged as the most prevalent comorbidity associated with pediatric CRS (26.9%).20 A hypothesis is that allergic inflammation might cause chronic obstruction of osteomeatal complex. Although their relationship remains controversial, children with CRS should be screened for allergies.21 Indeed, AR is characterized by IgE-mediated inflammation after exposure to allergen in atopic individuals, causing rhinorrhea, nasal obstruction, nasal itching, and sneezing that are reversible spontaneously or with treatment. These symptoms occur during two or more consecutive days.22 Different from AR, a clinical consensus statement defined pediatric CRS as at least 90 continuous days of two or more symptoms of purulent rhinorrhea, nasal obstruction, facial pressure/pain, or cough and either endoscopic signs of mucosal edema, purulent drainage, or nasal polyposis and/or CT scan changes showing mucosal changes within the osteomeatal complex and/or sinuses.23
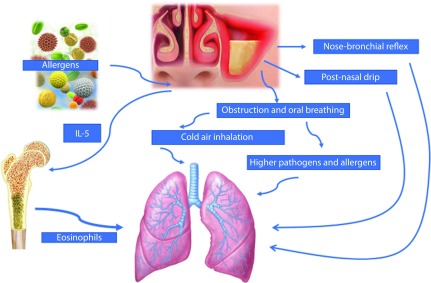
The link between CRS and asthma has been widely investigated. Around 40% of children with asthma show radiological or endoscopic signs of rhinosinusitis.24,25 It is still debated whether CRS is causative of asthma or if it is a part of United Airway Disease. Local mechanisms, that have been suggested, include nose-bronchial reflex, postnasal drip with inflammatory mediators spreading to the lower airways, and impaired sinus function. Sinonasal inflammation leads to obstruction and oral breathing and allows cold air, higher amounts of pathogens, and allergens to reach the lower airways, as well as the propagation of inflammation by both contiguity and the bloodstream. These effects might be responsible for epithelial barrier impairment and triggering bronchial constriction.26 This interaction is complicated by the fact that the clinical expression of upper airway disease varies with age. A higher number of asthma symptoms are seen in children aged 6–10 years old who have been diagnosed with rhinosinusitis by endoscopy compared to other age groups of children. This is probably due to the progressive development of the paranasal sinuses during childhood.27
Phenotypes and endotypes
At the beginning of the 20th century, Wilhelm Johannsen introduced the term ‘phenotype’ as ‘the observable structural and functional characteristics of an organism determined by its genotype and modulated by its environment.’ AADs are heterogeneous and have been classified into different phenotypes based on the different clinical expressions of the disease. Advances in molecular biology have allowed for the investigation of AAD pathophysiology through the identification of endotypes. An endotype consists of the underlying molecular mechanisms that are involved in the phenotypic expression of the disease. The accurate identification and characterization of endotypes is essential to set up targeted therapies.28,29
Phenotypes and endotypes of AR
AR can be classified according to the causal allergen into two main phenotypes: seasonal (SAR) or perennial (PAR). Its pathophysiology is mainly mediated by IgE with eosinophilic infiltration. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines revision from 2001 introduced a classification as intermittent or persistent AR based on the duration of symptoms (less or more than 4 weeks or 4 days per week). These terms are not synonymous with SAR and PAR. In fact, patients with SAR may have persistent symptoms, and patients with PAR may have intermittent symptoms.30 The revision of the ARIA guidelines in 2016 kept the classifications of SAR and PAR. Severity classification ranges from mild to severe forms according to the symptoms.31 Local AR (LAR) is a new phenotype proposed for AR, and LAR is characterized by a lack of systemic atopy evidence, positive response in the nasal allergen provocation test, and local IgE production in the nasal mucosa].32
A 10-year study follow-up confirmed that LAR is an independent phenotype, and conversion to systemic atopy was not significant compared to controls (9.7 versus 7.8%; p=0.623).33 Interestingly, LAR worsens and increases the risk of suffering from asthma.33 Polyallergic patients with AR experienced significantly more severe nasal symptoms than monoallergic ones and showed higher infiltration of eosinophilic and inflammatory mast cells in nasal cytology. Thus, the inflammatory pattern in nasal cytology may be a useful instrument to better characterize the phenotypes and endotypes of allergic and nonallergic rhinitis and realize personalized treatments.34
Phenotypes and endotypes of CRS
CRS is probably the least investigated AAD and has a low volume of published research, although it has high prevalence.35 The two main phenotypes described are CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).36 Nasal polyposis is rare in childhood and might be a ‘red flag’ for systemic diseases, such as cystic fibrosis or primary ciliary dyskinesia. An Italian monocentric study reported 56 pediatric patients with nasal polyposis over a 17-year period, with 32% showing positive skin prick tests.37
A case control study on 173 patients, a tissue cluster analysis of inflammatory biomarkers, identified ten cluster endotypes. Four of them had predominant noneosinophilic inflammation; six had eosinophilic inflammation, of which three were characterized by high IL-5, eosinophilic cationic protein, and serum IgE expression; and three had intermediate levels of IL-5. It was found that IL-5 levels correlated with CRSwNP and increased prevalence of asthma.38
Phenotypes and endotypes of asthma
Asthma is the most heterogeneous among AADs, and its complexity can be summarized by the definition of ‘asthma syndrome.’ Asthma classification is based on the presence of atopy, comorbidities, triggering factors (exercise, drugs, and smoke), response to treatment, and inflammatory patterns. Hence, the different phenotypes are early-onset allergic, late-onset eosinophilic, exercise-induced, obesity-related, and neutrophilic asthma.39 The main endotypes of asthma include eosinophilic asthma (Th2-high) and neutrophilic or pauci-granulocytic asthma (Th2-low) according to the inflammatory pattern, induced sputum, bronchoalveolar lavage, or blood cell count.40
The Th2-high endotype is the most common in children, and it is often associated with early-onset severe asthma, atopy, and responsiveness to steroids. Th2-high cytokine pattern includes the so-called ‘alarmins’: IL-33, IL-25, and thymic stromal lymphopoietin. ‘Alarmins’ have been suggested to start eosinophilic inflammation and increase IL-5, IL-4, and IL-13 expression. These cytokines lead to the recruitment of eosinophils, mast cells, and basophils, as well as Ig-E synthesis. The cytokines involved in Th2-low asthma are IL-8, IL-17A, and IL-22, and this type of asthma is characterized by a neutrophilic or paucigranulocytic infiltrate. T2-low asthma is usually associated with severe and corticosteroid-resistant asthma.41
Definition and identification of validated biomarkers in AAD
Biomarkers are needed in precision medicine to achieve the goals of personalized treatment. A biomarker may be considered as ‘a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention, including therapeutic interventions.’ An ideal biomarker should be noninvasive, objective, reproducible, cost effective, and predictive of treatment response. They can serve as diagnostic, monitoring, predictive, treatment-response, or prognostic biomarkers.42 Biomarkers provide an accurate characterization of endotypes that allows the treatment of selected subsets of patients with drugs acting selectively on a specific pathway. Indeed, biological drugs are labeled only for patients who fulfill strict criteria (e.g., eosinophils blood count for anti-IL5) – that is, the right drug for the right patient.
Role of IgE in AR, CRS, and asthma
IgE plays a key role in AAD. Its secretion is modulated by B cells after the switch from IgG to IgE induced by Th2 cytokines. It is a marker of atopy and allergen sensitization and acts as an early-phase effector of the Th2 hypersensitivity immune response. IgE represents a therapeutic target for anti-IgE drugs.43 High serum levels of total IgE represent a diagnostic biomarker for AAD. Total IgE is associated with bronchial hyperreactivity as well.44 Serum-specific IgE is a predictive biomarker of AR,39,45 and total and serum IgE correlate with asthma severity.46 Its role as a predictive biomarker of treatment response is questioned, but it is a reliable monitoring biomarker of the response to anti-IgE therapies.47 Regarding AIT, serum-specific IgE is not predictive or a monitoring biomarker, although it represents a valid biomarker to select patients for treatment with AIT. The serum IgE/total IgE ratio may be a potential predictive biomarker in AIT.42,48
Role of eosinophils in AR, CRS, and asthma
Eosinophils are involved in high-Th2 inflammation, and induced sputum eosinophil count ≥3% is the gold standard as a diagnostic biomarker for eosinophilic airway inflammation. Nevertheless, their routine measurement is limited by the invasiveness of the process and the difficulties to obtain sputum, especially in children.49 Sputum eosinophils correlate with disease severity. Blood eosinophils may represent an alternative, reliable, noninvasive biomarker that shows good correlation with the percentage of sputum eosinophils in asthma.50
Blood eosinophil count correlates with severe asthma and increased exacerbation rates if the count is ≥300 cells/μL.46 Furthermore, blood eosinophil counts ≥300 cells/μL in patients with asthma are a predictive and monitoring biomarker of the response to biological therapies, especially anti-IL-5 drugs. The decrease in blood eosinophils can also serve as a monitoring biomarker during treatment with anti-IL-5.51 The retrospective Next Steps Toward personalised care: EvaLuating responders to XoLAIR treatment in patients with SAA (STELLAIR) study questioned the role of blood eosinophils as a predictive biomarker of the response to omalizumab and found similar response rates and reductions in exacerbation rates, regardless of blood eosinophil counts.52 In CRS, nasal and blood eosinophils are markers of CRSwNP, more severe disease, and the prediction of the response to corticosteroids, especially in patients from Western countries compared with Asians. It has been hypothesized because of a combination of both genetic and environmental factors.53,54
Role of fractional exhaled nitric oxide (FeNO) and other emerging biomarkers in AR, CRS, and asthma
Inducible nitric oxide synthase produces nitric oxide (NO) as a bronchodilator in airways. FeNO is a biomarker of eosinophilic inflammation. High FeNO in patients with AR is associated with bronchial hyperreactivity and future risk of the development of asthma.55 Furthermore, elevated FeNO levels are predictive of good treatment response to corticosteroids.56 Nevertheless, the role of FeNO in the management of asthma still remains controversial.57 Emerging biomarkers include serum periostin,58,59 dipeptidyl peptidase-4 (DDP-4),60 eotaxin 1 and its receptor CCR3,61 exhaled breath condensate (EBC),62 volatile organic compounds (VOCs),62 YKL-40,63 and high mobility group box 1 (HMGB1).64–66 However, new emerging biomarkers need more investigations for standardization and validation.
Therapeutic targets in UAD during childhood
AIT in AR in children
The management of AR based on allergen avoidance and symptomatic drugs (antihistamines, intranasal corticosteroids, and leukotriene-receptor antagonists) is often suboptimal and characterized by inadequate symptom control and side effects, especially for PAR. AIT represents the best option in the treatment of AR. It is the only disease-modifying therapy and is indicated in moderate-to-severe intermittent or persistent AR.67,68 It consists of low, increasing doses of allergen administration by a subcutaneous (SCIT) or sublingual (SLIT) route, and the effects usually last at least 3 years. This treatment modulates the immune system, particularly T regulator cells (T-regs), which induces tolerance to a specific allergen. T-regs seem to be defective in subjects with AR.69
Indeed, T-regs produce anti-inflammatory cytokines IL-10 and TGF-β. AIT has been found to increase IL-10 synthesis, which reduces Th2 cytokines (IL-13, IL-4, and IL-5), reduces eosinophil activation, and promotes IgG4 production.70 A recent review summarized previous Cochrane reviews, systematic reviews, meta-analyses, and randomized clinical trials on AIT.71 The study evidenced the efficacy and safety of both SCIT and SLIT in reducing AR symptoms and medication use and inducing long-term remission. The heterogeneity and small sample size of previous studies represented a limit in establishing the superiority of SCIT or SLIT.71
Long-term effectiveness was reported for symptoms and quality of life after 3 years of treatment and 2 years after discontinuation of house dust mite (HDM) SCIT in both children and adults with AR, but the effect was greater in children.72 However, SLIT is easier and better tolerated than SCIT in children. After the first dose, the following doses can be administered at home. Poddighe and colleagues analyzed studies on SLIT in children.73 SLIT for grass pollen rhinitis was shown to be effective, particularly if started preseasonally. However, evidence of the efficacy of SLIT in HDM-AR is less strong than in grass pollen rhinitis because of the small number and scale of randomized controlled trials available.73
The safety and effectiveness of SLIT versus a placebo have been confirmed in a Cochrane systematic review.74 The side effects are usually limited to the upper airways or gastrointestinal tract. No patients with anaphylaxis were reported in the included studies, although isolated patients with anaphylaxis, who were almost all individuals with previous severe reactions to SCIT, were described. Thus, it might represent a valid and low risk alternative to SCIT.74
Local nasal immunotherapy is another alternative and safe route of administration, by means of spray or dry powder, although its effectiveness is lower compared with SCIT and SLIT in patients with allergic rhinitis and comorbid asthma. Local nasal immunotherapy seems to have a local effect only on nasal symptoms.75
To reduce the risk of systemic adverse reactions associated with SCIT, physically or chemically modified allergens, called allergoids, have also been tested, showing both efficacy and reduced IgE-binding capacity.76 Nevertheless, there are few comparative studies between allergens and allergoids as immunotherapy.77
Anti IgE in AR and CRS
IgE is crucial in the pathophysiology of AAD, which makes it a potential therapeutic target. Omalizumab is a humanized IgG1 monoclonal antibody that binds to free IgE, thus preventing interaction with its receptors (Fc epsilon RI) and downregulating its expression by dendritic cells and mast cells.78 Its use in AR treatment is still off-label, and its application in upper airway disease and asthma has been tested in several randomized clinical trials.79 A multicenter randomized double-blind placebo-controlled trial examined 536 adults with moderate-to-severe SAR, and symptom severity significantly improved in the subgroup treated with 300 mg of omalizumab every 3 or 4 weeks compared to placebo.80 A meta-analysis of 11 studies on 2870 patients with moderate-to-severe AR reported a statistically significant reduction in symptom score. However, the accuracy was limited by the different prevalence of comorbidities among studies.81
A combination of omalizumab and AIT contributed to improved symptom scores in 221 children with SAR and adolescents compared to AIT plus placebo.82 Improvements were also seen in sinonasal symptoms and nasal polyps in a patient with refractory CRSwNP and comorbid asthma, which suggested that omalizumab may also have a role as a target therapy in CRS (particularly CRSwNP), where IgE is involved.83 A systematic review analyzed two randomized clinical trials on the use of anti-IgE in comparison to a placebo for CRS treatment. One study included 23 adults with CRS and comorbid asthma. The authors concluded that there is little evidence for the efficacy of anti-IgE as a treatment for CRS, especially for quality of life. Indeed, only one of the two studies showed a significant reduction in symptoms, endoscopic scores, and radiological scores.84–86 Further randomized clinical trials with larger samples and on populations of children are needed.
Anti-IL-5 in CRS
IL-5 is a cytokine involved in Th2 eosinophilic/high inflammation. It recruits, activates, and promotes the survival of eosinophils. CRSwNP has been associated with eosinophilic inflammation, but mainly in Caucasian subjects. Anti-IL5 drugs are currently approved only for asthma and include mepolizumab, and reslizumab, that targets circulating IL-5, and benralizumab, that binds to IL-5 receptors. Evidence has been reported for the effectiveness and safety of anti-IL5 drugs on refractory nasal polyposis but is limited to three trials. In a randomized double-blind controlled trial, reslizumab (3 mg/kg, intravenous) was effective in reducing the size of nasal polyps.87 Two months of treatment with intravenous mepolizumab (750 mg) improved endoscopic and imaging scores in patients with refractory nasal polyposis.88 A randomized double-blind controlled trial examined 105 adults with severe recurrent nasal polyposis, and monthly intravenous mepolizumab (750 mg) significantly reduced the need for surgery at 25 weeks, endoscopic nasal scores, and symptom scores compared to placebo.89
Therapeutic targets in childhood asthma
AIT in controlled asthma in children
The Global Initiative for Asthma (GINA) guidelines introduced AIT as an add-on therapy for HDM-sensitized adults affected by AA and comorbid AR. Its use is restricted to patients with partially controlled mild-to-moderate AA and FEV1≥70% predicted because of the high risk of systemic adverse reactions in those with uncontrolled asthma.90 The effectiveness and safety of AIT have been assessed in both adults and children with AA, but few studies have been performed with children.91–93
A systematic review reported a reduction in asthma medication use for SCIT and improved quality of life in patients aged ≤18 years old. However, evidence was stronger for SCIT than SLIT.94 Regarding SLIT, a meta-analysis found a reduction in symptom scores and medication use in pediatric AA, although the studies analyzed were heterogeneous.95 Furthermore, SLIT demonstrated long-term effectiveness at 5 years after discontinuation in 60 children with asthma and AR due to HDM.96
A recent review used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for AIT.97 However, the reviewed studies suffered from heterogeneity, high risk of bias, and lack of standardization for outcomes. The authors stated that there is currently little evidence to recommend AIT in childhood AA until further well-designed randomized clinical trials provide higher strength of evidence about its beneficial effects.97 Interesting data have been obtained from the combination of SCIT and omalizumab, which might increase the efficacy of AIT and allow for restrictions on AIT to be overcome in patients with uncontrolled asthma.98 In a retrospective study on 90 children, combined therapy with AA reduced the occurrence of systemic reactions in comparison to SCIT alone.99
Several studies have hypothesized that there is a disease-modifying effect of AIT that reduces the risk of developing new sensitizations and asthma in children, but they were limited to small groups.93 Two large studies were performed: the prevention of allergy (PAT) study and the grass tablet asthma prevention (GAP) study. The PAT study enrolled 205 children with allergies to grass or birch pollen allergy, who were randomized to receive SCIT for 3 years. The treatment group had significantly lower risk of developing asthma and fewer asthma symptoms compared to the controls at the end of 3 years of treatment and at the 5- and 10-year follow-up.100–102 As treatment to prevent the development of new sensitizations, AIT is controversial, and evidence is limited to short-term periods. The reduction in the risk of onset of the first allergic disease was not significant. Further studies are needed to evaluate long-term effects.
Anti-IgE in severe AA during childhood
The pathophysiology of AA is strictly related to IgE, which is involved in the early and late phases of the inflammatory response. There is evidence that blocking free IgE interferes with the inflammatory cascade, which was the rationale to develop and test the humanized anti-IgE monoclonal antibody omalizumab.103 In 2001, two phase III trials were conducted with 1071 adolescents and adults with moderate-to-severe asthma, which demonstrated that omalizumab significantly reduced asthma exacerbations, symptoms, ICS, and the use of rescue medications compared with the placebo group. Improvement in pulmonary function test was also observed.104,105 Milgrom and colleagues performed a randomized double-blind placebo-controlled trial on 334 children with asthma controlled by ICS, which showed that omalizumab led to a significant decrease in asthma exacerbations (18.2 versus 38.5%) and ICS used, but there was no significant improvement in spirometric parameters. No serious adverse reactions were reported.106,107
Thus, omalizumab was first approved by the Food and Drug Administration (FDA) in 2003 for asthma treatment at the age of 12 and up, and its use was extended to children in 2009 by the European Medicines Agency (EMA) and in 2016 by the FDA. It is indicated as an add-on therapy for patients aged 6 years or older with moderate-to-severe persistent AA who have symptoms that are not controlled with common medications, positive skin test reactions, or IgE specific to a perennial aeroallergen and total IgE of 30–1500 IU/mL. The additional criteria of the EMA are frequent daytime symptoms or nighttime awakenings along with multiple documented severe asthma exacerbations, despite daily high-dose ICS, plus a long-acting inhaled β2-agonist. Reduced lung function (FEV1<80% predicted) is an additional condition for patients aged ≥12 years. The frequency of subcutaneous administration (monthly or twice a month) and dosage are guided by total IgE levels and weight.108–110
Recently, a multicenter Italian study tested omalizumab in 47 children and adolescents suffering from severe AA. After 12 months of treatment, the rates of exacerbation were reduced by 91% (p<0.001). Furthermore, there were significant reductions in hospitalization rates and the use of oral and ICS compared with the previous year. FEV1 improvement was not significant, however, which was probably due to the young age of the patients, who already had FEV1>80% at baseline.111
There have been concerns about omalizumab in relation to both immediate and long-term adverse events, particularly the risk of anaphylaxis and malignancy, respectively. Postmarket data have provided consistent evidence of its safety profile. Anaphylaxis was reported in 0.2% of treated patients, which is similar to the incidence of anaphylaxis in the general population. In controlled studies, the incidence of anaphylaxis was 0.14 versus 0.07% for the controls.112 The safety profile has been confirmed in children with asthma as well, where no differences in adverse events were observed in comparison with a placebo.113 Data on the safety and effectiveness of omalizumab have led to an increasing number of trials to assess its efficacy in different pediatric IgE-mediated diseases, such as AR, nasal polyposis, severe refractory atopic dermatitis, allergic bronchopulmonary aspergillosis, food allergy, and anaphylaxis. Omalizumab is licensed as an add-on therapy for the treatment of chronic spontaneous urticaria in adolescents aged >12 years who have an inadequate response to H1-antihistamine treatment.114–116
The duration of treatment with omalizumab still remains a debated issue. It is a long-term treatment because it requires about 12 weeks to show any clinical improvement. Therefore, its effectiveness should be evaluated at 16 weeks of treatment, defined as primary endpoint by clinical trials and summary of product characteristics to continue the treatment.108,109 A further assessment might be considered at 52 weeks of treatment.117 A multicenter study in France assessed the effects of the immediate discontinuation of omalizumab in 35 children who had previously received a 24-month course of omalizumab and no severe exacerbation during the previous year. Asthma control worsened in only 8 out of 35 patients after 3–17 months. The 8 patients with poor control were also more atopic. This suggests omalizumab might have a potential role as a disease-modifying drug and preventing airway remodeling, probably when treatment is started in childhood. Nevertheless, further prospective studies on large samples are required.118
Anti-IL-5 in severe eosinophilic asthma during childhood
IL-5 regulates eosinophilic inflammation and is thus a therapeutic target in eosinophilic asthma.119 Mepolizumab is an anti-IL-5 drug that is indicated as an add-on maintenance treatment for patients with severe refractory eosinophilic asthma aged 12 years and older (100 mg SC). The Dose Ranging Efficacy And Safety With Mepolizumab in Severe Asthma (DREAM) study, a double-blind placebo-controlled trial, evaluated the efficacy and safety of three doses (75, 250, and 750 mg) of intravenous mepolizumab every 4 weeks compared with placebo over a 52-week treatment period in subjects with severe uncontrolled refractory asthma. This study confirmed the effectiveness of mepolizumab in reducing exacerbation rates in patients with severe refractory eosinophilic asthma (621 adolescents and adults). Serum eosinophil counts fell.120–122 The response to treatment is positively correlated with a blood eosinophil count of at least 150 cells/μL.123 Similar results were obtained with 36 children, which led the EMA to license it for children aged 6–11 years old (40 mg).124 Another study examined a small group of seven adolescents with severe eosinophilic asthma who were not eligible for omalizumab or nonresponders to it. Twelve months of treatment with mepolizumab reduced the severity and rates of asthma exacerbations and blood eosinophils. However, mepolizumab did not improve symptom scores or lung function test results.125
Benralizumab is a monoclonal antibody directed against the α-chain of the IL-5 receptor (CD125). An analysis of the Efficacy and Safety Study of Benralizumab Added to High-dose Inhaled Corticosteroid Plus LABA in Patients With Uncontrolled Asthma (SIROCCO) and Efficacy and Safety of benralizumab in Asthmatic Adults and Adolescents Inadequately Controlled on Inhaled Corticosteroid Plus Long-acting β2 Agonist (CALIMA) phase III studies showed a statistically significant reduction in asthma exacerbations (46%) and improved FEV1 in adults with severe eosinophilic asthma (blood eosinophil counts >300 cells/μL) when treated with benralizumab every 8 weeks for a year, regardless of IgE levels.126 Currently, there are no ongoing trials testing benralizumab in children. Reslizumab is a humanized monoclonal antibody to IL-5 that leads to a decrease in the production and maturation of eosinophils. This drug is indicated in severe uncontrolled eosinophilic asthma in patients aged >18 years old. Treatment with reslizumab at 3 mg/kg intravenously every 4 weeks significantly improved FEV1 and symptom scores. Treatment for 1 year markedly improved FEV1 and reduced asthma exacerbations and symptom scores versus a placebo in patients with severe eosinophilic asthma and comorbid self-reported CRSwNP.127
In 2017, a Cochrane review analyzed 13 randomized double-blind placebo-controlled clinical trials regarding the effects of anti-IL5 on 6000 individuals aged more than 12 years old, who mainly had severe eosinophilic asthma. Anti-IL-5 drugs reduced blood eosinophil counts and significantly reduced asthma exacerbation rates. Lung function improvement was significant but small. No serious adverse events were reported. Interestingly, benralizumab reduced exacerbation in noneosinophilc asthma as well.128
Future opportunities in therapy for AAD in children
Research on biologic drugs for the treatment of AAD is rapidly expanding, and experimentation with new molecules in the coming years will be able to further enrich the therapeutic alternatives available to reference specialists.129 Regarding the future of anti-IgE therapy, it is appropriate to point out that a new monoclonal antibody, ligelizumab (anti-IgE-mAb), is currently being tested. This drug has shown an affinity for human IgE that is approximately 50 times higher in comparison to omalizumab, as well as a nine-fold increase in the suppression power of circulating free IgE levels.130 From a clinical point of view, ligelizumab showed greater efficacy than omalizumab in the asthmatic response to inhalant allergens.131
In addition to anti-IgE monoclonal antibodies, a new category of drugs is currently being tested, which have a molecular target that is located further upstream than the direct blockade of circulating IgE. This category of monoclonal antibodies is defined as anti-CemXmAb and provides an alternative mechanism of intervention for the IgE-mediated allergic inflammatory pathway. They bind to IgE expressed on the membrane of B IgE-switched lymphoblasts, which causes their lysis and thus prevents the allergen-mediated generation of IgE-producing plasma cells.132
Moreover, these biologic drugs do not bind to free IgE, and consequently, their action is independent of serum IgE levels. However, it should be noted that quilizumab, which belongs to this category of drugs, has not had any appreciable clinical benefit in adults with AA that is not controlled by standard therapy.133 In addition, one of the most interesting areas in development involves the therapeutic vaccines that are able to trigger the immune system to produce therapeutic anti-IgE antibodies. This approach could potentially provide a further step forward in the treatment of allergic diseases.134
Regarding the new horizons of therapy with anti-IL-4 and IL-13 biologics, the only drug that has achieved satisfactory clinical evidence to date in efficacy and safety is dupilumab, which is an anti-IL-4 receptor monoclonal antibody that blocks both IL-4 and IL-13 signaling. These effects were confirmed by the results of two clinical trials (VENTURE and QUEST).135,136 These multicenter studies examined 210 and 1902 patients, respectively, over 12 years old with steroid-dependent or uncontrolled asthma, and the results showed that dupilumab is effective in improving symptom control and respiratory function. Subsequently, in 2018, dupilumab received FDA approval as add-on treatment for moderate-to-severe asthma in patients aged 12 years or older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma.137 In February 2019, the EMA approved it for severe asthma from the age of 12, adding raised blood eosinophils and/or raised FeNO as criteria.138 An ongoing trial (NCT03560466) is evaluating dupilumab in children with asthma.139 In the treatment of asthma, a potential and cheaper alternative therapteutic option to biologics may be represented by drugs, acting on airway inflammation, such as mast cells stabilizers (e.g., pemirolast) and tetomilast, a phosphodiesterase 4 inhibitor.140–142 Nevertheless, few trials, limited to adults with asthma or chronic obstructive pulmonary disease, respectively, have been performed. There is an ongoing trial to evaluate tetomilast in chronic obstructive pulmonary disease associated with emphysema. Further studies are needed.140–142
Conclusions
In the field of allergic diseases and the era of the ‘omics’ sciences, we are increasingly turning to precision-tailored patient-specific medicine. The range of choices is wide and spans from the use of standard therapy to the use of new biologic drugs. Among these, omalizumab and, recently, mepolizumab are available for the pediatric populations (Table 1). New molecules such as reslizumab, benralizumab, ligelizumab, and dupilumab are currently being studied. To optimize treatment with biologic drugs in pediatric allergies, it is necessary to identify and validate biomarkers that are able to identify patients with particular severity characteristics, as well as to define the optimal duration of the treatment and directly compare between the various biologic treatments available.
Table 1.
Summary of novel biologics in AAD.
| Mechanism of action | Indications | Administration | |
|---|---|---|---|
| Omalizumab | Anti-IgE |
|
Subcutaneously q2 or q4 weeks (dosage depending on weight and IgE levels) |
| Ligelizumab | Anti-IgE |
|
Subcutaneously q2 weeks (240 mg) |
| Mepolizumab | Anti-IL5 |
|
Subcutaneously every 4 weeks (100 mg ≥12 years; 40 mg 6–11 years) |
| Benralizumab | Anti-IL5 receptor |
|
Subcutaneously (30 mg q4 weeks for the first three doses, q8 weeks thereafter) |
| Reslizumab | Anti-IL5 |
|
Intravenous infusion q4 weeks (dosage depending on weight) |
| Dupilumab | Anti-IL4 and IL-13 |
|
Subcutaneously q week (first dose 600 mg then 300 mg) |
Acknowledgements
None.
Footnotes
Contributions: AL developed the original idea and the final revision, AG and GFP wrote the manuscript, GP and CC revised firstly the manuscript and contributed to English revision and references update, and CS, GLM, and SL made the final analysis and critical revision of the manuscript. All authors read and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/06/dic.212590-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Giallongo A, Parisi GF, Licari A, Pulvirenti G, Cuppari C, Salpietro C, Marseglia GL, Leonardia S. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/novel-therapeutic-targets-for-allergic-airway-disease-in-children/
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 3 May 2019
References
- 1.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372:2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 2.Pols DH, Wartna JB, van Alphen EI, et al. Interrelationships between atopic disorders in children: a meta-analysis based on ISAAC questionnaires. PLoS One. 2015;10:e0131869. doi: 10.1371/journal.pone.0131869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: definition and limits of asthma control in the pediatric population. Front Pediatr. 2018;6:170. doi: 10.3389/fped.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Licari A, Marseglia GL. Current and future challenges in pediatric severe asthma. Curr Med Res Opin. 2018;34:943–944. doi: 10.1080/03007995.2018.1439463. [DOI] [PubMed] [Google Scholar]
- 5.Shah R, Saltoun CA. Chapter 14: acute severe asthma (status asthmaticus) Allergy Asthma Proc. 2012 May-Jun;33(Suppl 1):47–50. doi: 10.2500/aap.2012.33.3547. [DOI] [PubMed] [Google Scholar]
- 6.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 7.Licari A, Castagnoli R, Denicolò CF, Rossini L, Marseglia A, Marseglia GL. The nose and the lung: united airway disease? Front Pediatr. 2017;5:44. doi: 10.3389/fped.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons FE. Allergic rhinobronchitis: the asthma-allergic rhinitis link. J Allergy Clin Immunol. 1999;104:534–540. doi: 10.1016/S0091-6749(99)70320-9. [DOI] [PubMed] [Google Scholar]
- 9.Sehmi R, Wood LJ, Watson R, et al. Allergen-induced increases in IL-5 receptor alpha-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. Am J Respir Crit Care Med. 2001 Oct 15;164(8 Pt 1):1403–1409. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allakhverdi Z, Comeau MR, Smith DE, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrand TS, Sprenger JD, Agosti JM, Longton GM, Witherspoon RP, Henderson WR., Jr Long-term acquisition of allergen-specific IgE and asthma following allogeneic bone marrow transplantation from allergic donors. Blood. 2004;104:3086–3090. doi: 10.1182/blood-2004-05-1775. [DOI] [PubMed] [Google Scholar]
- 12.Passalacqua G, Ciprandi G, Canonica GW. United airways disease: therapeutic aspects. Thorax. 2000;55(Suppl 2):S26–S27. doi: 10.1136/thorax.55.suppl_2.S26. https://dx.doi.org/10.1136%2Fthorax.55.suppl_2.S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leynaert B, Neukirch C, Kony S, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004;113:86–93. doi: 10.1016/j.jaci.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson J, Bjerg A, Lötvall J, et al. Rhinitis phenotypes correlate with different symptom presentation and risk factor patterns of asthma. Respir Med. 2011;105:1611–1621. doi: 10.1016/j.rmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi C, Passalacqua G, Gargioni S, et al. The natural history of respiratory allergy: a follow-up study of 99 patients up to 10 years. Respir Med. 2001;95:9–12. doi: 10.1053/rmed.2000.0945. [DOI] [PubMed] [Google Scholar]
- 16.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–1057. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 17.Marseglia GL, Merli P, Caimmi D, et al. Nasal disease and asthma. Int J Immunopathol Pharmacol. 2011;24:7–12. doi: 10.1177/03946320110240S402. [DOI] [PubMed] [Google Scholar]
- 18.Ciprandi G, Cirillo I, Pistorio A. Impact of allergic rhinitis on asthma: effects on spirometric parameters. Allergy. 2008;63:255–260. doi: 10.1111/j.1398-9995.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 19.Marseglia GL, Cirillo I, Vizzaccaro A, et al. Role of forced expiratory flow at 25-75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007;28:74–8. doi: 10.2500/aap.2007.28.2920. [DOI] [PubMed] [Google Scholar]
- 20.Sedaghat AR, Phipatanakul W, Cunningham MJ. Prevalence of and associations with allergic rhinitis in children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2014;78(2):343–347. doi: 10.1016/j.ijporl.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgalas C, Vlastos I, Picavet V, van Drunen C, Garas G, Prokopakis E. Is chronic rhinosinusitis related to allergic rhinitis in adults and children? Applying epidemiological guidelines for causation. Allergy. 2014 Jul;69(7):828–833. doi: 10.1111/all.12413. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;86:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 23.Brietzke SE, Shin JJ, Choi S, et al. Clinical consensus statement: pediatric chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;151(4):542–553. doi: 10.1177/0194599814549302. [DOI] [PubMed] [Google Scholar]
- 24.Poddighe D, Brambilla I, Licari A, Marseglia GL. Pediatric rhinosinusitis and asthma. Respir Med. 2018;14:94–99. doi: 10.1016/j.rmed.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Licari A, Brambilla I, De Filippo M, Poddighe D, Castagnoli R, Marseglia GL. The role of upper airway pathology as a co-morbidity in severe asthma. Expert Rev Respir Med. 2017;11:855–865. doi: 10.1080/17476348.2017.1381564. [DOI] [PubMed] [Google Scholar]
- 26.Licari A, Caimmi S, Bosa L, Marseglia A, Marseglia GL, Caimmi D. Rhinosinusitis and asthma: a very long engagement. Int J Immunopathol Pharmacol. 2014;27:499–508. doi: 10.1177/039463201402700405. [DOI] [PubMed] [Google Scholar]
- 27.Marseglia GL, Castellazzi AM, Licari A, et al. Inflammation of paranasal sinuses: the clinical pattern is age-dependent. Pediatr Allergy Immunol. 2007;18:10–12. doi: 10.1111/j.1399-3038.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 28.Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- 29.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Saleh HA, Durham SR. Perennial rhinitis. BMJ. 2007;335:502–507. doi: 10.1136/bmj.39304.678194.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 32.Rondón C, Canto G, Blanca M. Local allergic rhinitis: a new entity, characterization and further studies. Curr Opin Allergy Clin Immunol. 2010;10:1–7. doi: 10.1097/ACI.0b013e328334f5fb. [DOI] [PubMed] [Google Scholar]
- 33.Rondon C, Campo P, Eguiluz-Gracia I, et al. Local allergic rhinitis is an independent rhinitis phenotype: the results of a 10-year follow-up study. Allergy. 2018;73:470–478. doi: 10.1111/all.13272. [DOI] [PubMed] [Google Scholar]
- 34.Gelardi M, Landi M, Ciprandi G. The pragmatic role of nasal cytology: a point-of-care testing to implement precision medicine in clinical practice. Rev Alerg Mex. 2018;65:259–263. doi: 10.29262/ram.v65i3.373. [DOI] [PubMed] [Google Scholar]
- 35.Rudmik L. Chronic rhinosinusitis: an under-researched epidemic. J Otolaryngol Head Neck Surg. 2015;44:11. doi: 10.1186/s40463-015-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caimmi D, Matti E, Pelizzo G, et al. Nasal polyposis in children. J Biol Regul Homeost Agents. 2012;26:S77–S83. [PubMed] [Google Scholar]
- 38.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 40.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Licari A, Castagnoli R, Brambilla I, et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol. 2018;31:44–55. doi: 10.1089/ped.2018.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzpatrick AM. Biomarkers of asthma and allergic airway diseases. Ann Allergy Asthma Immunol. 2015;115:335–340. doi: 10.1016/j.anai.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Ciprandi G, Marseglia GL, Castagnoli R, et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. 2015;11:1321–1333. doi: 10.1586/1744666X.2015.1086645. [DOI] [PubMed] [Google Scholar]
- 44.Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 45.Marinho S, Simpson A, Söderström L, et al. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007;62:1379–1386. doi: 10.1111/j.1398-9995.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 46.Konradsen JR, Skantz E, Nordlund B, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol. 2015;26:772–779. doi: 10.1111/pai.12457. [DOI] [PubMed] [Google Scholar]
- 47.Bousquet J, Rabe K, Humbert M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101:1483–1492. doi: 10.1016/j.rmed.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Licari A, Castagnoli R, Brambilla I, et al. Biomarkers of immunotherapy response in patients with allergic rhinitis. Expert Rev Clin Immunol. 2018;14:657–663. doi: 10.1080/1744666X.2018.1504679. [DOI] [PubMed] [Google Scholar]
- 49.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 50.Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115–120. doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 51.Kansal P, Nandan D, Agarwal S, et al. Correlation of induced sputum eosinophil levels with clinical parameters in mild and moderate persistent asthma in children aged 7-18 years. J Asthma. 2018;55:385–390. doi: 10.1080/02770903.2017.1338725. [DOI] [PubMed] [Google Scholar]
- 52.Humbert M, Taillé C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51 doi: 10.1183/13993003.02523-2017. pii: 1702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Workman AD, Kohanski MA, Cohen NA. Biomarkers in chronic rhinosinusitis with nasal polyps. Immunol Allergy Clin North Am. 2018;38:679–692. doi: 10.1016/j.iac.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Ciprandi G, Gallo F, Ricciardolo FL, Cirillo I. Fractional exhaled nitric oxide: a potential biomarker in allergic rhinitis? Int Arch Allergy Immunol. 2017;172:99–105. doi: 10.1159/000456548. [DOI] [PubMed] [Google Scholar]
- 56.Smith AD, Cowan JO, Brassett KP, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172:453–459. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 57.Gomersal T, Harnan S, Essat M, et al. A systematic review of fractional exhaled nitric oxide in the routine management of childhood asthma. Pediatr Pulmonol. 2016;51:316–328. doi: 10.1002/ppul.23371. [DOI] [PubMed] [Google Scholar]
- 58.Song JS, You JS, Jeong SI, et al. Serum periostin levels correlate with airway hyperresponsiveness to methacholine and mannitol in children with asthma. Allergy. 2015;70:674–681. doi: 10.1111/all.12599. [DOI] [PubMed] [Google Scholar]
- 59.Inoue T, Akashi K, Watanabe M, et al. Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr Allergy Immunol. 2016;27:521–526. doi: 10.1111/pai.12575. [DOI] [PubMed] [Google Scholar]
- 60.Shiobara T, Chibana K, Watanabe T, et al. Dipeptidyl peptidase-4 is highly expressed in bronchial epithelial cells of untreated asthma and it increases cell proliferation along with fibronectin production in airway constitutive cells. Respir Res. 2016;17:28. doi: 10.1186/s12931-016-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada T, Miyabe Y, Ueki S, et al. Eotaxin-3 as a plasma biomarker for mucosal eosinophil infiltration in chronic rhinosinusitis. Front Immunol. 2019;10:74. doi: 10.3389/fimmu.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Mastrigt E, de Jongste JC, Pijnenburg MW. The analysis of volatile organic compounds in exhaled breath and biomarkers in exhaled breath condensate in children - clinical tools or scientific toys? Clin Exp Allergy. 2015;45:1170–1188. doi: 10.1111/cea.12454. [DOI] [PubMed] [Google Scholar]
- 63.Leonardi S, Filippelli M, Lanzafame A, et al. Serum ykl-40 in children with asthma. J Biol Regul Homeost Agents. 2015;29:114–119. [PubMed] [Google Scholar]
- 64.Manti S, Leonardi S, Parisi GF, et al. Focus on pleiotropic role of HMGB1 in the onset of allergic and non-allergic respiratory diseases. Curr Respir Med Rev. 2017;13:1–5. doi: 10.2174/1573398X13666170529113627. [DOI] [Google Scholar]
- 65.Chirico V, Lacquaniti A, Salpietro V, et al. High mobility group box 1 (HMGB1) in childhood: from bench to bedside. Eur J Pediatr. 2014;173:1123–1136. doi: 10.1007/s00431-014-2327-1. [DOI] [PubMed] [Google Scholar]
- 66.Salpietro C, Cuppari C, Grasso L, et al. Nasal high-mobility group box-1 protein in children with allergic rhinitis. Int Arch Allergy Immunol. 2013;161:116–121. doi: 10.1159/000345246. [DOI] [PubMed] [Google Scholar]
- 67.Licari A, Castagnoli R, Bottino C, Marseglia A, Marseglia G, Ciprandi G. Emerging drugs for the treatment of perennial allergic rhinitis. Expert Opin Emerg Drugs. 2016;21:57–67. doi: 10.1517/14728214.2016.1139082. [DOI] [PubMed] [Google Scholar]
- 68.Licari A, Ciprandi G, Marseglia A, et al. Current recommendations and emerging options for the treatment of allergic rhinitis. Expert Rev Clin Immunol. 2014;10:1337–1347. doi: 10.1586/1744666X.2014.955476. [DOI] [PubMed] [Google Scholar]
- 69.Romagnani S. Regulatory T cells: which role in the pathogenesis and treatment of allergic disorders? Allergy. 2006;61:3–14. doi: 10.1111/j.1398-9995.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 70.Ciprandi G, Fenoglio D, Cirillo I, et al. Sublingual immunotherapy: an update on immunologic and functional effects. Allergy Asthma Proc. 2007;28:40–3. doi: 10.2500/aap.2007.28.2974. [DOI] [PubMed] [Google Scholar]
- 71.Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339–349.e10. doi: 10.1016/j.jaci.2015.12.1298. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Wang C, Cao F, et al. Comparison of long-term efficacy of subcutaneous immunotherapy in pediatric and adult patients with allergic rhinitis. Allergy Asthma Immunol Res. 2019;11:68–78. doi: 10.4168/aair.2019.11.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poddighe D, Licari A, Caimmi S, et al. Sublingual immunotherapy for pediatric allergic rhinitis: the clinical evidence. World J Clin Pediatr. 2016;5:47–56. doi: 10.5409/wjcp.v5.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT) Allergy. 2011;66:740–752. doi: 10.1111/j.1398-9995.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 75.Passalacqua G, Canonica GV. Local nasal specific immunotherapy for allergic rhinitis. Allergy Asthma Clin Immunol. 2006;2:117–123. doi: 10.1186/1710-1492-2-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen NT, Raskopf E, Shah-Hosseini K, Zadoyan G, Mösges R. A review of allergoid immunotherapy: is cat allergy a suitable target? Immunotherapy. 2016;8:331–349. doi: 10.2217/imt.15.121. [DOI] [PubMed] [Google Scholar]
- 77.Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Ann Allergy Asthma Immunol. 2018;121:293–305. doi: 10.1016/j.anai.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Licari A, Marseglia A, Caimmi S, et al. Emerging and future therapies for allergic rhinitis. J Biol Regul Homeost Agents. 2015;29:38–46. [PubMed] [Google Scholar]
- 79.Licari A, Castagnoli R, Panfili E, et al. An update on anti-IgE therapy in pediatric respiratory diseases. Curr Respir Med Rev. 2017;13:22–29. doi: 10.2174/1573398X13666170616110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casale TB, Condemi J, LaForce C, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–2967. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- 81.Tsabouri S, Tseretopoulou X, Priftis K, et al. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–340.e1. doi: 10.1016/j.jaip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:274–280. doi: 10.1067/mai.2002.121949. [DOI] [PubMed] [Google Scholar]
- 83.Grundmann SA, Hemfort PB, Luger TA, et al. Anti-IgE (omalizumab): a new therapeutic approach for chronic rhinosinusitis. J Allergy Clin Immunol. 2008;121:257–258. doi: 10.1016/j.jaci.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 84.Pinto JM, Mehta N, DiTineo M, et al. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010;48:318–324. doi: 10.4193/Rhin09.144. [DOI] [PubMed] [Google Scholar]
- 85.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116.e1. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 86.Hong CJ, Tsang AC, Quinn JG, et al. Anti-IgE monoclonal antibody therapy for the treatment of chronic rhinosinusitis: a systematic review. Syst Rev. 2015;4:166. doi: 10.1186/s13643-015-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 88.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995.e1–e8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 89.Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031.e14. doi: 10.1016/j.jaci.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 90.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2016. [Accessed April 1st, 2019]. http://www.ginasthma.org.
- 91.Asamoah F, Kakourou A, Dhami S, et al. Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:25. doi: 10.1186/s13601-017-0160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27:1–35. doi: 10.18176/jiaci.0149. [DOI] [PubMed] [Google Scholar]
- 93.Tosca MA, Licari A, Olcese R, et al. Immunotherapy and asthma in children. Front Pediatr. 2018;6:231. doi: 10.3389/fped.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rice JL, Diette GB, Suarez-Cuervo C, et al. Allergen-specific immunotherapy in the treatment of pediatric asthma: a systematic review. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3833. pii: e20173833. [DOI] [PubMed] [Google Scholar]
- 95.Penagos M, Passalacqua G, Compalati E, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008;133:599–609. doi: 10.1378/chest.06-1425. [DOI] [PubMed] [Google Scholar]
- 96.Di Rienzo V, Marcucci F, Puccinelli P, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–210. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 97.van de Griendt EJ, Tuut MK, de Groot H, et al. Applicability of evidence from previous systematic reviews on immunotherapy in current practice of childhood asthma treatment: a GRADE (Grading of Recommendations Assessment, Development and Evaluation) systematic review. BMJ Open. 2017;7:e016326. doi: 10.1136/bmjopen-2017-016326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braido F, Corsico A, Rogkakou A, et al. The relationship between allergen immunotherapy and omalizumab for treating asthma. Expert Rev Respir Med. 2015 Apr;9(2):129–34. doi: 10.1586/17476348.2015.1000866. [DOI] [PubMed] [Google Scholar]
- 99.Har D, Lee MJ. Systemic reaction rates with omalizumab, subcutaneous immunotherapy, and combination therapy in children with allergic asthma. Allergy Asthma Proc. 2019;40:35–40. doi: 10.2500/aap.2019.40.4173. [DOI] [PubMed] [Google Scholar]
- 100.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 101.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 102.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 103.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 104.Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 105.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 106.Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 107.Licari A, Marseglia G, Castagnoli R, et al. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov. 2015;10(9):1033–1042. doi: 10.1517/17460441.2015.1048220. [DOI] [PubMed] [Google Scholar]
- 108.Poddighe D, Brambilla I, Licari A, et al. Omalizumab in the therapy of pediatric asthma. Recent Pat Inflamm Allergy Drug Discov. 2018;12(2):103–109. doi: 10.2174/1872213X12666180430161351. [DOI] [PubMed] [Google Scholar]
- 109.European Medicines Agency. Xolair. [Accessed April 1st, 2019]. https://www.ema.europa.eu/en/medicines/human/EPAR/xolair.
- 110.FDA Medication Data. Xolair Medication Guide. [Accessed April 1st, 2019]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf.
- 111.Licari A, Castagnoli R, Denicolò C, et al. Omalizumab in children with severe allergic asthma: the Italian real-life experience. Curr Respir Med Rev. 2017;13:36–42. doi: 10.2174/1573398X13666170426094536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788–797. doi: 10.1111/j.1365-2222.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 113.Chipps BE, Lanier B, Milgrom H, et al. Omalizumab in children with uncontrolled allergic asthma: review of clinical trial and real-world experience. J Allergy Clin Immunol. 2017;139:1431–1444. doi: 10.1016/j.jaci.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Licari A, Marseglia A, Caimmi S, et al. Omalizumab in children. Paediatr Drugs. 2014;16:491–502. doi: 10.1007/s40272-014-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parisi GF, Papale M, Giovanna Tardino L, et al. Omalizumab treatment in a 12 year-old girl with chronic spontaneous urticaria. J Dermatolog Treat. 2018 Nov;21:1–6. doi: 10.1080/09546634.2018.1551609. [DOI] [PubMed] [Google Scholar]
- 116.Parisi GF, Portale A, Papale M, et al. Successful treatment with omalizumab of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis: case reports and literature review. J Allergy Clin Immunol Pract. 2019 Feb 15; doi: 10.1016/j.jaip.2019.01.056. pii: S2213-2198(19)30165-5. http://dx.doi.org/S2213-2198(19)30165-5. [DOI] [PubMed] [Google Scholar]
- 117.Lai T, Wang S, Xu Z, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015 Feb 3;5:8191. doi: 10.1038/srep08191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deschildre A, Roussel J, Drumez E, et al. Omalizumab discontinuation in children with severe allergic asthma: an observational real-life study. Allergy. 2018 Nov 27; doi: 10.1111/all.13678. [DOI] [PubMed] [Google Scholar]
- 119.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 120.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 122.FDA Medication Data. Nucala Medication Guide. [Accessed April 1st, 2019]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf.
- 123.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556. doi: 10.1016/S2213-2600(16)30031-5. http://dx.doi.org/0.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 124.European Medicines Agency. Nucala Assessment Report. [Accessed April 1st, 2019]. https://www.ema.europa.eu/en/documents/variation-report/nucala-h-c-3860-ii-0013-g-epar-assessment-report-variation_en.pdf.
- 125.Weir E, Paton J. Mepolizumab in adolescents with severe eosinophilic asthma not eligible for omalizumab: one center’s early clinical experience. J Asthma. 2019 Feb;22:1–4. doi: 10.1080/02770903.2019.1579833. [DOI] [PubMed] [Google Scholar]
- 126.Chipps BE, Newbold P, Hirsch I, et al. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120:504–511. doi: 10.1016/j.anai.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 127.Weinstein SF, Katial RK, Bardin P, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self-reported chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2019;7:589–596. doi: 10.1016/j.jaip.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 128.Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017 Sep 21;9:CD010834. doi: 10.1002/14651858.CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Licari A, Castagnoli R, Brambilla I, et al. New approaches for identifying and testing potential new anti-asthma agents. Expert Opin Drug Discov. 2018;13:51–63. doi: 10.1080/17460441.2018.1396315. [DOI] [PubMed] [Google Scholar]
- 130.Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44:1371–1385. doi: 10.1111/cea.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gauvreau GM, Arm JP, Boulet LP, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. 2016;138:1051–1059. doi: 10.1016/j.jaci.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 132.Liour SS, Tom A, Chan YH, et al. Treating IgE-mediated diseases via targeting IgE-expressing B cells using an anti-CemX antibody. Pediatr Allergy Immunol. 2016;27:446–451. doi: 10.1111/pai.12584. [DOI] [PubMed] [Google Scholar]
- 133.Harris JM, Maciuca R, Bradley MS, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res. 2016;17:29. doi: 10.1186/s12931-016-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Licari A, Castagnoli R, De Sando E, et al. Development of a peptide conjugate vaccine for inducing therapeutic anti-IgE antibodies. Expert Opin Biol Ther. 2017;17:429–434. doi: 10.1080/14712598.2017.1289172. [DOI] [PubMed] [Google Scholar]
- 135.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 136.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 137.FDA Approved Drug Products. Dupilumab. [Accessed April 1st, 2019]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761055.
- 138.European Medicines Agency. Dupixent. [Accessed April 1st, 2019]. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/dupixent.
- 139.Clinical Trials.gov. Assessment of the safety and efficacy of dupilumab in children with asthma (liberty asthma excursion) [Accessed April 1st, 2019]. https://clinicaltrials.gov/ct2/show/NCT03560466.
- 140.Clinical Trials.gov. Safety and Efficacy of CRD007 in Adult Asthma Subjects. [Accessed April 1, 2019]. https://clinicaltrials.gov/ct2/show/NCT02615080.
- 141.Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking? Br J Pharmacol. 2008;155:288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clinical Trials. gov. Pilot Study of Tetomilast in Chronic Obstructive Pulmonary Disease (COPD) Associated With Emphysema (EMPHASIS) [Accessed April 1, 2019]. https://clinicaltrials.gov/ct2/show/NCT00874497.